The Feasibility of Enzyme Immunoassay Tests in the Absence of a Conventional Source of Electricity
Abstract
Background of the Study
African countries are facing frequent blackout. Thus in sub Saharan region, due to frequent power cut, the laboratory professionals find sometimes difficulty to carry out earlier diverse diagnostic tests.
Objective
The aim of this work is to evaluate the feasibility of enzyme immunoassay tests in the absence of a conventional source of electricity.
Methods
We developed a battery-powered experimental device, which was then applied to diagnose measles. The samples included 45 sera randomly selected from non-haemolysed serum samples received and stored at the National Public Health Laboratory of Benin. The experimental device is composed of two devices (Devices 1 and 2). The Device 1 provided an average temperature of 34.47 °C, 20 min after starting. With Device 2 an average temperature of 20.32 °C is obtained 15 min after starting.
Results
With the experimental device the same rate of measles antibody-positive sera (44.68%) was obtained as recorded from the test using the standard equipment of laboratory. The experimental device detected 18 negative and 8 intermediate results against respectively 19 and 7 by the standard equipment. The analysis of the results of both equipments shows a concordance rate of 93.33% with a kappa reproducibility coefficient of 0.89.
Conclusion
The device conceived in our study is a simply equipment allowing the realization of the enzyme immunoassay tests, in this case the ELISA anti-measles test. The rate of concordance obtained shows that this device can be used with commercial kits and at temperatures close to those recommended by the manufacturer without altering the results.
Author Contributions
Academic Editor: Tawfique AlZubiery, Associate professor in clinical Microbiology, Department of medical laboratory Faculty Medical and Health Sciences. Taiz University Al-Turbah branch.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Camille S.U. DOSSOU
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
In the last decades, Sub-Saharan region of Africa has faced daily energy failure. Laboratory technicians as well as workers in other sector, who work even at a public center experienced power outages some days. But the frequency of power outages is especially acute for rural professional. Indeed, compared to urban cities, majorities of sub-Saharan Africans workers are without electricity in rural areas1. The power cut has not only a socioeconomic impacts but also affect greatly the health care system. Indeed, Lin et al.2 have found that mortality and respiratory hospital admissions in New York city have increased two- to eightfold during the blackout in August 14th and 15th, 2003. Otherwise, during the power cut occurred in Zanzibar (Tanzania) from May 21st-June 18th, 2008, Burlando3 demonstrated that children exposed during the first month of pregnancy have significantly lower birth weights on average, by 45 grams. Furthermore, the same authors showed that significant reductions in weight for children exposed to the blackout in the fifth month of gestation.
In Benin, we observed that being off the electrical grid has also an impact on health facilities. However, private biomedical laboratories are by far the most likely in country to have difficulty to access electricity. Blackouts are often caused by unstable infrastructure that is likely to suffer from breaking and theft. This is not without consequences for electrical apparatus and therefore is a handicap for biologists in the context of biological diagnosis. However, in the context of the reporting and notification of mandatory reported disease, a biological diagnosis is required as soon as possible in order of the control of likely epidemic outbreak and to take appropriate measures to contain it. During the blackout, laboratory professionals faced difficulties to complete such diagnosis , since the power cut caused disruptions to health services due to the absence of standby power3,4.
In common laboratories, the diagnosis of certain mandatory reported diseases is done by the Enzyme Linked ImmunoSorbent Assay (ELISA). It is well known that ELISA is a gold standard technique performed for the laboratory diagnosis, based on the detection and quantification of the specific antibodies or antigens5. This technique is usually run with commercial kit according to manufacturer’s instructions. The steps of the analysis included some incubation at 37 °C and those at room temperature (often 25 °C)5,6. Thus, in many laboratories, these temperatures are obtained by the electrical equipment such as the water baths (for temperature of 37 °C) and the air conditioner (room temperature). However, none of these two temperatures, indicated by the manufacturer, is obtained in the conditions of power failure as most of Sub-Saharan Africa countries’ electricity supply is unreliable4.
Thus in this work, we evaluated the feasibility of ELISA tests in the absence of a conventional source of electricity. The objective was to replace the heat source (the oven) by another alternative source: incandescent lamps powered by batteries within a cardboard box, and on the other hand replace the source of room temperature (the air conditioner) by a cold accumulator. This experimental device was then tested by applying to the diagnosis of measles at the national public health laboratory of Benin.
Materials and Methods
This is a cross-sectional study carried out from January 9th to June11th, 2013.
Ethical Consideration
Ethical approval was obtained from the National Ethic Committee of the Ministry of Health while consents were obtained from the suspected measles patients and the relatives.
Development of the Experimental Device
The first device (Dispositif 1) is composed of a hermetic cardboard box (33 cm x 25 cm x 20 cm) containing another smaller cardboard, in which a space is reserved for the solid support of the ELISA test (a plastic tray containing a thin layer of wet compress). The tray is framed by two incandescent lamps of six (06) volts each, powered by eight (08) batteries of 1.5 volts at the rate of four (04) batteries per lamp. One of the lamps has in its circuit a switch acting as a thermostat, for manual regulation of the temperature inside the device (Figure 1).
Figure 1.Experimental device 1 designed to replace the oven used in standard equipment. Device 1 seen from the outside (A) and from inside (B), composed of a cardboard of dimension 33cm x 25cm x 20cm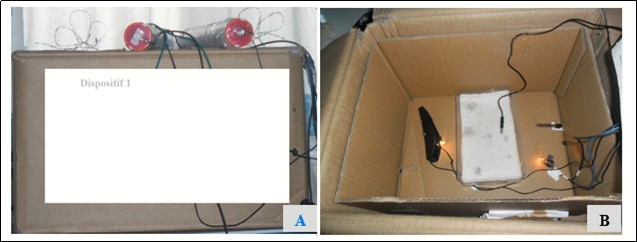
The second device (Dispositif 2) consists of a plastic box, hermetic and opaque (22 cm x 14 cm x 8 cm) containing a ice pack already in a state of charge at the time of power cut. The ice pack is covered with a layer of carded cotton on which will be deposited the solid support of the test (Figure 2).
Figure 2.Experimental device 2 designed to substitute the room temperature provider. Device 2 seen from the outside (A) and from inside (B), composed of a plastic, hermetic and opaque box of size 22cm x 14cm x 8cm containing an ice pack.
A probe thermometer incorporated in both devices makes it possible to follow the variation of the temperature.
Testing of Experimental Device
The devices conceived are applied for measles diagnosis.
Samples Collection
The study samples are composed of forty five (45) sera randomly selected from the non-haemolyzed serum samples, received at the national public health laboratory of Benin during the study period. These sera came from blood samples collected from patients suspected of measles from the seventy seven (77) districts of Benin. After collection, blood samples were reached the national laboratory within a period of three (03) days, where they were kept between 2°C and 8°C until they were tested. After testing, these samples were conserved at -30°C.
ELISA Assay
Testing was performed using indirect ELISA method. Anti-measles Virus IgM was detected using ELISA Kits manufactured by Siemens Healthcare Diagnostics product GmbH, Germany. All the samples from suspected patients were tested for measles specific IgM antibodies in 96 wells microplate. Since immunoglobulin G (IgG) can interfere in the detection of IgM by solid phase ELISA,7 tests were performed using 1/41 diluted serum in the rheumatoid factor absorbent (supply by Siemens), to precipitate IgG. A well sensitized with the antigen of the measles virus and a well sensitized with the control antigen were used for each sample. Each assay includes a negative control, a positive-strong control (provided by the manufacturer) and a positive-weak control (home-made). The assays were performed following the manufacturer’s instructions with slight modifications of temperature conditions. Briefly, 150 µL of each negative, positive control and diluted sera samples were distributed into the respective wells of the microtiter plate. The plate was covered with aluminium foil and incubated for 60 min at 37 °C. The incubation solution was discarded and the plate was washed 3 times with 300 µL/well of diluted wash buffer. The excess solution was removing by tapping the inverted plate on a paper towel. 100 µL of diluted Enzyme Conjugate Anti-IgM humaine/POD were added into each well. The plate was covered with a new aluminium foil and incubated again at 37 °C for 60 min. After washing the plate, 100 µL of TMB substrate solution were added into each well. The plate was then incubated in the dark at room temperature (25 °C) for 30 min. The substrate reaction was stopped by adding 100 µL of TMB stop solution into each well. Color changed from blue to yellow. The optical density was read at 450 nm using the microplate reader Multiskan EX, Shanghai (China).
Anti-Measles Virus Detection Using Standard Equipment
During this stage of the study, the numeric database and the epidemiological surveillance archives for measles, from the National Laboratory of Public Health, were consulted. The serological status with respect to measles and the adjusted optical densities of the 45 specimens selected were then recorded. The results recorded here were previously obtained with the same ELISA test kits described above which was done using the standard laboratory equipment and conditions. Here, the incubation temperatures were provided by the electric apparatus: the water bath (for 37 °C incubation) and the air conditioner (for 25 °C incubation).
Data Analysis
The results were recorded as adjusted optical density (ΔDO). According to the manufacturer's instructions, the results should be interpreted as follow: negative (DO ≤ 0.1), positive (DO ≥ 0.2), and undetermined (0.1<DO< 0.2). The Student's test and Chi-squared test of independence were used to compare respectively the ΔDOs and the proportions of serological status obtained from both the standard equipment and the experimental device. The analysis were done through the R software packages “Stats”8.
Results
Experimental Device Working
The first device noticed as “Device 1” (Figure 1) designed to replace the oven, displayed temperatures range from 27.90 °C to 35.70 °C with an average of 34.47 °C (95% CI = 34.05-34.89). This average temperature is obtained 20 minutes after starting the device and remains stable for 4 hours (Figure 3).
Figure 3.Temperature evolution curve obtained with Device 1 over the time. It provides a temperature of 34 °C ± 2 °C, against 37 °C±1 °C recommended for the ELISA kit.
In addition, for the second device which was designed to provide temperatures in range of room temperature, it displayed temperatures that varied between 18.50 °C and 23.40 °C with an average of 20.32 °C (95% CI = 19.94-20.69). This average temperature is obtained 15 minutes after starting the device and remains close to this average for 3 hours (Figure 4).
Figure 4.Temperature evolution curve provided by the device 2 over time. This device provides a temperature of 20 °C ± 2° C against 18 to 25 °C recommended.
Device Testing
After the design of the experimental device, it was used to diagnose the 45 specimens again. This is to evaluate the detection power of true positives. The results showed that the optical densities obtained ranged from -0.004 to 0.339 with an average of 0.1598 (95% CI = 0.125-0.194) (Figure 5). The device diagnosed 44.68% positive, a negative percentage of 38.30% and 17.02% of indeterminate (Table 1). In addition, the contingency table (Table 2) show a concordance rate of the serological statuses diagnosed with the standard equipment and the experimental device of 93.33% with a kappa reproducibility coefficient of 0.89.
Figure 5.Variation of the adjusted optical densities of each sample recorded using the standard equipment and the experimental device.
| IgM detection | Indeterminate | Negative | Positive | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Standard equipment | 7 | 14.89 | 19 | 40.43 | 21 | 44.68 | 47 | 100 |
| Experimental device | 8 | 17.02 | 18 | 38.30 | 21 | 44.68 | 47 | 100 |
| Experimental device | Total | ||||
| Positive | Indeterminate | Negative | |||
| Standard equipment | Positive | 20 | 01 | 00 | 21 |
| Indeterminate | 01 | 06 | 00 | 07 | |
| Negative | 00 | 01 | 18 | 19 | |
| Total | 21 | 08 | 18 | 47 | |
Comparison of the Device Results with those Obtained with the Standard Equipment.
This phase of the study focused on the retrospective analysis of data from 45 serum samples previously diagnosed for measles, using the Enzygnost anti-measles Virus/IgM® ELISA kit with a standard equipment. The data were collected from the numeric database and the epidemiological surveillance archive of the laboratory for comparison to those obtained with the experimental device. It appears that the recorded optical densities (OD) ranged from -0.011 to 0.385 with an average of 0.1567 (95% CI = 0.11-0.19) (Fig. 5). As shown in Table 1, 44.68% of positive, 40.43% of negative and 14.89% of indeterminate measles serological status were reported.
Discussion
The experimental device designed to replace the standard equipment using current power supply has provided temperatures close to those recommended for ELISA assay. The temperature provided by the Device 1 (34 °C) confirms the possibility of carrying out the incubations of the samples and the conjugate at a temperature close to 37 °C ± 1 °C, as indicated recommended. In addition, the temperatures provided by the Device 2 (up to 20 °C) allow absorption of the rheumatoid factor (RF) and incubation of the substrate under required temperature conditions (between 18 °C and 25 °C).
The experimental device shows almost the same adjusted optical densities (ΔDO) as the standard equipment (an average of 0.1598 vs. 0.1567). Indeed, there is no significant difference between the adjusted optical densities (ΔDO) recorded (p> 0.05). With both design device and standard equipment, almost the same level of serological status is detected. The kappa reproducibility coefficient (0.89) indicates a complete agreement between the results obtained during the two phases of the study. This suggest that the device we design here provide a range of temperatures required to perform ELISA test in case of power cut.
In order to improve the assay performance, instead of freshly coated plates, precoated plates can be used. Indeed it was found out that precoated plates will contribute to improved assay robustness at an acceptable diagnostic proficiency9 . Also, aluminum foil could be used to cover the inner surfaces of the cardboard containers which can act as a heat conduction to assist heat transfer through all facets of the inner container. Further, using a small battery-powered fan could better ensure the temperature distribution inside the inner container. Moreover, an extended polystyrene foam could be used as insulation between the inner and outer containers to prevent temperature transfer exchange between the inner and outer environment. Given the results obtained from the device testing, it would be interesting to consider with design engineers, more modern material than the carton. Moreover, the power mode of the device could be replaced by solar energy instead of batteries. However, the measure of samples optical densities requires microplate reader apparatus. In this study, we have not use an alternative device for the microplate reader. Thus the optical densities record can be done during power blackout only if there is UPS battery backup supplier in the lab. Then it is important to evaluate how long the antigen-antibody interaction is stable before reading the optical densities, using the experimental device.
We find overall a great rate of measles antibody-positive sera. But, the number of sample in lower to establish an accurate measles incidence in the country. Nevertheless, this percentage of positive samples mimic the measles epidemiological trend in Africa regions. Indeed, in 2016, the number of reported measles cases in Africa region was 36,269.10 But this trend has highly decreased compared to 2015 estimates. Despite the existence of a safe and effective vaccine, measles remains a major cause of morbidity and mortality11. The number of measles death estimated in 2016 in Africa was 37,50010. Therefore, the achievement of measles elimination goals, necessitates case-based surveillance including laboratory confirmation of suspect cases12. The confirmation necessitates also apparatus that work permanently. In this context, such experimental device conceived here need to be improved, mostly to help laboratory professionals in rural area where blackout frequently occurred.
The number of equivocal results (indeterminate specimens) are statistically significant compared to positive percentage (p<0.05). These samples should be retested in another reference laboratory or with another ELISA kit. In addition, another samples should be collected after 3-4 weeks and retested for IgM antibodies13. Furthermore, the same sera samples can be tested for IgG antibodies. This latter can only be done if the individual has not received a recent measles vaccination14. Indeed, it is reported that positive IgM alone does not confirm a measles case15. The test kit we used in the case of this study requires to precipitate IgG since they interfere with IgM detection. This kind of commercial kits are the standard test for the rapid laboratory diagnosis of measles. But these assays can lead to both false-positive and false-negative results16. Some commercial version of the measles IgM capture enzyme immunoassay kits do not require the removal of IgG antibodies and are considered to be more specific than the indirect immunoassays for detection of measles IgM antibodies17. Thus, such kits can be used as a second test kit for assessing equivocal sera samples.
Conclusion
Mandatory reported diseases carry challenges especially to developing countries, where the technical platform for diagnosis is sometimes lacking, and in addition there are power cuts. It is then essential to develop a palliative equipment to avoid disturbances in the course of tests during power cuts. Thus the device designed in our study is a simple equipment allowing the realization of ELISA tests, apply to the anti-measles ELISA test. The concordance rate obtained shows that this device can be used with commercial kits and at temperatures close to those recommended by the manufacturer without altering the results. However, the batteries used to power the equipment could be replaced by a 12-volt battery, powered by a suitable solar panel.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Interest
None.
Acknowledgements
The authors wish to pay tribute to their Lecturer Dr Evelyne Lozes (University of Abomey-Calavi, Benin), who was the intended corresponding authors and who passed away while this manuscript was under drafting.
References
- 1.Gallup. (2012) In Sub-Saharan Africa, Most Workers Are Without Electricity [Internet]. Available from: https://news.gallup.com/poll/151889/Sub-Saharan-Africa-Workers-Without-Electricity.aspx , Gallup.com
- 2.Lin S, Fletcher B A, Luo M, Chinery R, Hwang S-A. (2011) . Health Impact in New York City During the Northeastern Blackout of 2003. Public Health Rep.126(3): 384-93.
- 3.Burlando A. (2010) The Impact of Electricity on Work and Health: Evidence from a Blackout in Zanzibar. SSRN Electronic Journal.1–44 .
- 4.Eberhard A, Foster V, Briceño C, Ouedraogo F, Camos D. (2008) Underpowered: The State of the Power Sector in Sub-Saharan Africa.67.
- 5.Alhajj M, Farhana A. (2020) Enzyme Linked Immunosorbent Assay (ELISA). In: StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing;. Available from: http://www.ncbi.nlm.nih.gov/books/NBK555922/
- 6.Crowther J R. (2009) . The ELISA Guidebook [Internet].Totowa, NJ:Humana Press;2009 (cited 2020 Sep 16). (Walker JM, editor.Methods in Molecular Biology.516 .
- 7.Salonen E M, Vaheri A, Suni J, Wager O. (1980) Rheumatoid factor in acute viral infections: interference with determination of IgM, IgG, and IgA antibodies in an enzyme immunoassay. , J Infect 142(2), 250-5.
- 8.R Core Team. (2017) R: A language and environment for statistical computing.[Internet].Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/.
- 9.Rebeski D E, Winger E M, Robinson M M, Gabler C M, Dwinger R H. (2000) Evaluation of antigen-coating procedures of enzyme-linked immunosorbent assay method for detection of trypanosomal antibodies. Vet Parasitol.90(1–2): 1–13
- 10.Dabbagh A, Patel M K, Dumolard L, Gacic-Dobo M, Mulders M N et al. (2017) Progress Toward Regional Measles Elimination-Worldwide. , MMWR Morb Mortal Wkly 66(42), 1148-1153.
- 11.Coughlin M M, Beck A S, Bankamp B, Rota P A. (2017) . Perspective on Global Measles Epidemiology and Control and the Role of Novel Vaccination Strategies. Viruses.9(1): 1-17.
- 12.Rota P A, Moss W J, Takeda M, de Swart RL, Thompson K M. (2016) . Measles. Nat Rev Dis Primers.2: 16049.
- 13.Ballew H. (2000) Neutralization. In: Clinical virology manual. 3rd ed. Washington (DC): Spector S, Hodinka RL, Young SA 127-3.
- 14.Dietz V, Rota J, Izurieta H, Carrasco P, Bellini W. (2004) The laboratory confirmation of suspected measles cases in settings of low measles transmission: conclusions from the experience in the Americas. Bulletin of the World Health Organization. 88(11), 852-857.
- 15.Bolotin S, Lim G, Dang V, Crowcroft N, Gubbay J. (2017) The utility of measles and rubella IgM serology in an elimination setting. PLoS One.12(8):e0181172 , Ontario, Canada