Demonstration of the Capabilities of Transabdominal Ultrasonography in Assessment of Structures and Functional Disorders of Locally Advanced Gastric Cancer of Diverse Localization
Abstract
Introduction:
Ultrasound study of locally advanced gastric cancer that has spread to adjoining tissue and lymph nodes. This tumor can be associated with T2 to T4 stages of cancer. A “Locally advanced gastric cancer” is a tumor, which may be categorized as ‘resectable’ cancer when compared with M1 advanced cancer.
Objective:
The aim of this study was to evaluate the Capabilities of transabdominal ultrasonography in assessment of structures and functional disorders of the locally advanced gastric cancer of diverse localization
Materials and Methods:
A total of61 patients with locally advanced gastric cancer were analyzed of which 36 (59,0%) were males (mean age 62.7 years) and 25 (41,0%) were females (mean age 59.3 years). All patients were managed surgically and underwent preoperative X-ray, virtual gastroscopy techniques, multidetector computed tomography and transabdominal ultrasonography (USG).
Histopathology results found, in 58 (95,1%) cases adenocarcinoma, in 3 (4,9%) – ring-cell carcinoma (cricoidal) gastric cancer was established. Stage T2 was diagnosed in 16 (26.2%) cases, T3 - in 41 (67.2%) cases, T4 - in 4 (6.6%) cases. The stomach tumor in 29 (47.5%) cases was localized mainly in the antrum, 27 (44.3%) – in the body, 5 (8.2%) in the cardia and fundus (Table 1). In 24 (39,3%) cases, pyloric stenos was diagnosed - of which in 6 (9,8%) it was compensated, in 18 (29,5%) - sub compensated. All patients underwent preoperative X-ray, virtual gastroscopy techniques, multidetector computed tomography and transabdominal ultrasonography (USG). Normal ultrasound features were observed in 35 patients without gastric pathology. Ultrasonography was carried out with the convex and micro convex transducers in the frequency range of 2-5 MHz and 4-7 MHz respectively in B and color Doppler modes.
Results:
The polypoid type of gastric cancer was detected in 3 (4,9±2,8%) cases, the ulcerative type – in 18 (29,5±5,8%), the infiltrative ulcerative type – in 27 (44,3±6,4%) and the diffuse infiltrative type – in 13 (21,3%±5,2%) cases respectively. In 24 (39,3%) cases, pyloric stenos was diagnosed - of which in 6 (9,8%) it was compensated, in 18 (29,5%) – sub compensated. The layers of the gastric wall were not differentiated in all patients with sub compensated pyloric stenos. The gastric wall thickness of the affected area was 10,2±2,9mm in the case compensated pyloric stenosis, the length was 27,1±6,2mm, the diameter of the pylorus was 8,3±0,8mm. Among patients with sub compensated pyloric stenos, the thickness of the gastric wall was 19,8±4,1mm, the length was 43,6±4,5mm, the pyloric diameter was 4,3±1,1mm.
Among the 61 patients studied, pathological vascularization was detected in 42 (68.8%) cases. It was observed that, all 4 (6.5%) patients with gastric cancer were stage T4 and 38 (62.3%) were stage T3. Vascularization was weak in 13 cases, in 24 cases - moderate, and in 5 cases - enhanced.
Metastases to the regional lymph nodes were diagnosed in 52 cases. Ultrasonographically, they were detected only in 37 (71.2%) cases.
Conclusions:
In the diagnosis of locally advanced gastric cancer, ultrasonography demonstrates good capabilities for determining the extent and depth of the affected area. Color doppler mode allows the study of vascularisation of a locally thickened area, as well as nearby enlarged lymph nodes, which is very important to ascertain the degree of malignancy of the hyperplastic process. ltrasonography can independently determine the degree of pyloric stenosis in patients with distal gastric cancer.
Author Contributions
Academic Editor: Rongbiao Tang, Ruijin hospital, China
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Rizvan Yagubovich Abdullaiev et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Gastric carcinoma is the fourth most common cancer and the second leading incidence of cancer worldwide. Locally advanced cancer is that cancer which has spread from its point of origin to nearby tissue or lymph nodes. Treatment of gastric cancer remains an urgent problem. In recent years, some advances have been made in the field of adjuvant therapy for patients with locally advanced gastric cancer 1.
Gastric cancer is one of the most common malignant neoplasms and is the fifth leading cause of cancer mortality in western populations, despite its decreasing incidence in Europe and the United States 2. In the majority of patients, gastric cancer is usually diagnosed at a later stage of the disease progression when there are classic symptoms of weight loss, persistent dull pain in the epigastrium and loss of appetite. In 8% of cases he is detected in the form of early gastric cancer (EGC) during the mildly symptomatic or asymptomatic phases 3.
The severity of gastric cancer is associated with the depth of invasion (lesion depth) along the layers of the stomach wall, infiltration to regional lymph nodes and the presence of metastases to adjacent and distant organs (TNM classification). Depending on the degree of damage of the layers of the wall of the stomach the pathology can be classified into Early and Advanced gastric cancer (EGC and AGC) 4. Diagnosis of early gastric cancer in Western countries is 5-15%, when in Japan it reaches 30-50%. According to the results of 20 years of research Ahn XC (2011), EGC to progress to an advanced stage of takes about 8 months 5.
Literature definitions of Locally-advanced gastric cancer are controversial. Some researchers believe that AGC starts from T2, and others - from T3 6, 7, 8. According to Han S-K. (2012) ‘Locally advanced gastric cancer’ is a tumor of T4b (adjacent organ invasion) or inoperable tumour due to adjacent organ invasion or bulky LNs without distant metastasis. Another defines, ‘Locally advanced gastric cancer’ as a tumor, which may be a ‘resectable’ cancer when compared with M1 advanced ’cancer 9.
To determine the extent of Advanced gastric cancer Sol A.D. et al. (2014) analyzed the literature on this controversy. The results of the studies gave rise to the following conclusion 10: It is difficult to differentiate between resectable advanced stage, unresectable M0 and M1 cancers using the term ‘advanced’. It must be noted that ‘advanced gastric cancer’ may imply a number of stages of the disease and studies must highlight the exact clinical TNM classification for evaluation of the study.Accurate preoperative staging is therefore essential for optimal surgical management with consideration of preoperative and/or postoperative chemotherapy 11. The most commonly used technique for the staging of GC is multidetector computed tomography (MDCT) as it provides higher resolution images 12. The high efficiency of endoscopic ultrasonography (EUS) in assessing the depth of invasion is considered only in early gastric cancer (EGC) 13, 14.
Currently, the application of transabdominal ultrasonography in the diagnosis of gastric diseases is insignificant. However, in previously published articles, the capability of the method in differentiating the layers of the stomach wall, and to diagnose pyloric stenosis in cancer of the output section of the stomach is clearly demonstrated 15.
Objective
The aim of this study was to evaluate the capabilities of transabdominal ultrasonography in the assessment of structures and functional disorders of locally advanced gastric cancer of various locations.
Materials and Methods
The study design included 61 patients with locally advanced gastric cancer. 36 (59,0%) cases were males (mean age 62.7 years) and 25 (41,0%) females (mean age 59.3 years). All patients were on surgical treatment at the Kharkov Regional Oncology Center (Ukraine).
Histology results showed, in 58 (95,1%) cases adenocarcinoma, and in 3 (4,9%) – ring-cell carcinoma (cricoidal) gastric cancer was established. According to the classification by the American Joint Committee on Cancer (AJCC) 16, the stage of T2 was confirmed – in 16 (26,2%) cases, the stage of T3 – in 41 (67,2%) cases and the stage of T4 – in 4 (6,6%) cases . The diverse locations of gastric tumor was established as follows; in 29 (47.5%) cases antrum, in 27 (44,3%) cases – the body, in 5 (8.2%) cases- the cardia and fundus (Table 1).
Table 1. Location and stage of gastric cancer.| Stage of carcinoma | Antrum | Body | Cardia and fundus | Total |
|---|---|---|---|---|
| T2 | 5 (8,2±3,5%) | 9 (14,8±4,8%) | 2 (3,3±2,3%) | 16 (26,2±5,6%) |
| T3 | 23 (37,7±6,2%) | 16 (26,2±5,6%) | 2 (3,3±2,3%) | 41 (67,2±6,0%) |
| T4 | 1 (1,6±1,6%) | 2 (3,3±2,3%) | 1 (1,6±1,6%) | 4 (6,6±3,2%) |
| Total | 29 (47,5±6,4%) | 27 (44,3±6,4%) | 5 (8,2±3,5%) | 61 (100,0%±1,3%) |
All the patients underwent preoperative X-ray, virtual gastroscopy techniques, multidetector computed tomography (MDCT) and transabdominal ultrasonography (TAS).
Normal ultrasound features was shown in 35 patients without gastric pathology. TAS was carried out with the convex and microconvex transducers in the frequency range of 2-5 MHz and 4-7 MHz respectively in B and color Doppler modes.
Results
TAS of an empty stomach visualizes only the abdominal part of the esophagus which is 3-4 cm long. In the center of the esophagus, the mucosa of both walls is well defined as a thin hyperechoic linear structure. The walls of the unchanged esophagus have a linear layered structure (Figure 1).
Figure 1.Ultrasonography of the abdominal esophagus (vertical arrows). Horizontal arrow shows mucosa of the esophagus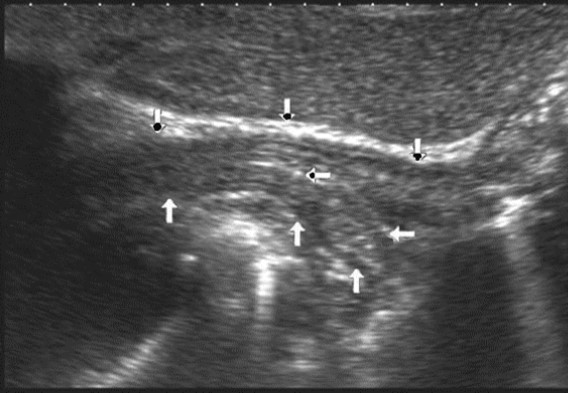
Immediately after contrasting, bubbles of gas appear in the cavity of the stomach, which gradually disappears. The thickness of the stomach wall in the region of the subcardiac part and the bottom is not more than 4 mm, the layers of the wall are not well differentiated, and there are no local thickenings (Figure 2).
Figure 2.Ultrasonography of the stomach. Vertical arrows show the fundus of the stomach, horizontal arrow – the papillary muscle of the left ventricle.
In the area of the body all the gastric wall layers are clearly differentiated. The gastric wall thickness when filled with the fluid does not exceed 3 mm (Figure 3). During peristaltic waves, there is also thickening of the gastric wall, which disappears after a few seconds. The greatest wall thickness of 3-5mm is observed in the antrum part. A careful monitoring illustrates the opening and closing of the pyloric canal (Figure 4).
Figure 3.Ultrasonography of the stomach body. The arrows show all layers of the stomach wall in the transverse section
Figure 4.Ultrasonography of the stomach. The vertical arrows show the anterior wall of the antrum; the horizontal arrows – pyloric, which is not fully open.
To diagnose a locally advanced gastric cancer, the following echographic symptoms were elicited:
1. Local thickening of the gastric wall more than 6 mm.
2. Deterioration or absence of differentiation of the layers of the gastric wall.
3. Decreased or absence of local peristaltic waves.
4. Presence of parietal formations protruding into the gastric cavity and outside peristaltic waves.
5. The maximum thickness and extent of the affected area of the stomach.
6. The maximum diameter of the pyloric canal during the passage of the peristaltic wave
The polypoid type of gastric cancer was observed in 3 (4,9±2,8%) cases, the ulcerative type – in 18 (29,5±5,8%), the infiltrative ulcerative type – in 27 (44,3±6,4%) and the diffuse infiltrative type – in 13 (21,3%±5,2%) cases respectively (Table 2). On the echogram, the tumor was
Visualized as a polypoid formation on a wide base above the gastric mucosa of a non-uniform structure, an irregular surface (Figure 5, Figure 6, Figure 7).
Table 2. Macroscopic type and stage of gastric cancer.| Stage of carcinoma | Macroscopic type оf tumor (n=61) | |||
| Polypoid | Ulcerative | Infiltrative ulcerative | Diffuse infiltrative | |
| T2 | 2 (3,3±2,3%) | 5 (8,2±3,5%) | 5 (8,2±3,5%) | 4 (6,6±3,2%) |
| T3 | 1 (1,6±1,6%) | 11 (18,0±4,9%) | 21 (34,4±6,1%) | 8 (13,1±4,3%) |
| T4 | - | 2 (3,3±2,3%) | 1 (1,6±1,6%) | 1 (1,6±1,6%) |
| Total | 3 (4,9±2,8%) | 18 (29,5±5,8%) | 27 (44,3±6,4%) | 13 (21,3%±5,2%) |
Figure 5.The polypoid type of gastric carcinomas for T3 stage. On the posterior wall of the antrum, a polypoid formation with a fuzzy contour is visualized, the image of the mucous and serous membranes (arrows) is discontinuous.
Figure 6.The gastric carcinoma for T3 stage. The tumor is visualized as a polypoid formation on a wide base above the gastric mucosa of a non-uniform structure, an irregular surface (arrows).
Figure 7.Endoscopic picture of gastric carcinoma for T3 stage. The tumor is visualized as a formation on a wide base above the gastric mucosa of a non-uniform structure, an irregular surface (arrows).
T2 stage of diffuse carcinoma was recorded in 14 cases – among them 5 cases of ulcerating forms, 5 cases of infiltrative ulcerative forms and 4 cases of diffuse infiltrative forms. Gastroscopy diagnosis was established in all 10 cases of ulcerative and infiltrative ulcerative forms of gastric carcinomas and in 3 cases of diffuse infiltrative forms. Ultrasound diagnosis was established in 13 cases of diffuse carcinoma, except for one case where the location of the ulcerative form was in the fundus of the stomach (Figure 8, Figure 9, Figure 10).
Figure 8.Diffuse infiltrative form of the gastric carcinoma of stage T2 on the anterior wall of the stomach (arrows). The integrity of the mucous layer is not broken.
Figure 9.The gastric carcinoma of diffuse form. An area with a local thickening up to 5 mm, a length of about 37 mm, is visualized on the anterior wall of the stomach body. The left arrows show the differentiated intact wall layers, the right arrows – local thickening hypoechoic of the anterior wall. Mucous, muscular and serous membranes are differentiated.
Figure 10.The gastric carcinoma of diffuse infiltrative form. An area with a local thickening up to 1,7 cm, a length of 3,68 cm, is visualized on the anterior wall of the antrum (arrows). The thickening of the normal stomach wall is 0,34 cm.
T3 stage of polypoid form was recorded in one case, ulcerative form – in 11 cases, infiltrative ulcerative forms – in 21 cases and diffused infiltrative forms – in 8 cases. The Sonographic features of infiltrative ulcerative form of gastric carcinoma was shown as local uneven thickening of the stomach wall with a length of up to several centimeters with intermittent images and ulceration of the mucosa (Figure 11). The Ulcerative form of gastric carcinoma was shown as a raised margins surrounded by a thickened gastric wall with irregular margins (Figure 12). Gastric carcinomas of diffuse infiltrative form is illustrated as a diffuse thickening of the muscle layer, a smooth contour, discontinuous mucosal image without ulceration and involvement of the serous membrane in the process (Figure 13).
Figure 11.Gastric carcinomas of ulcerating infiltrating forms in the anterior wall of the stomach body of stage T3.
Figure 12.Gastric carcinomas of ulcerating forms in the anterior wall of the stomach body of stage T3. Tumor is manifested with raised margins surrounded by a thickened gastric wall without clear margins.
Figure 13.Gastric carcinomas of diffuse infiltrative form in the posterior wall of the stomach body of stage T3. The tumor is manifested by diffuse thickening of the muscle layer, a smooth contour, discontinuous mucosal image without ulceration and involvement of the serous membrane in the process. An enlarged, altered lymph node of low echogenicity, round shape, without differentiation of the peripheral and central part, is visualized behind the stomach.
Distal gastric cancer was observed in 24 (39,3%) cases and contributed to the development of pyloric stenosis: in 6 (9,8%) cases it was compensated, in 18 (29,5%) – was sub compensated. The layers of the wall were not differentiated in all patients with sub compensated pyloric stenosis. In the case of compensated pyloric stenosis, the thickness of the affected area was 10,2±2,9mm, the length was 27,1±6,2mm, the diameter of the pylorus was 8,3±0,8mm (Figure 14). Among patients with sub compensated pyloric stenosis, the thickness of the stomach wall was 19,8±4,1mm, the length was 43,6±4,5mm, the pyloric diameter was 4,3±1,1mm (Figure 15).
Figure 14.Gastric carcinomas of diffuse infiltrative form in the atrium of T2 stage. Compensated pyloric stenos. On an empty stomach in the cavity of the stomach is determined an a small amount of fluid. The diameter of the pyloric canal more than 7 mm.
Figure 15.Distal gastric carcinomas of diffuse infiltrative form of T3 stage. Sub compensated pyloric stenos (arrows). The thickness of the anterior wall of the stomach is 9,13 mm, extent of the affected area – 7,84 cm. On an empty stomach in the cavity of the stomach is determined an a large amount of fluid. The diameter of the pyloric canal is about 6 mm.
Moschetta M. et al. (2011) suggested a new method for CT reconstruction which improves the diagnostic accuracy of the GC T-set 17. Vessel probe, initially developed for examination of small vessels can display images in orthogonal multiplanar, oblique and curved reconstructions as well as three-dimensional and curved reformat views. The vessel probe algorithm permits a more accurate view of gastric wall stratification due to the reduction of the partial volume artifact.
Color Doppler mode was used to assess the nature of the gastric wall lesion in pathologically thickened areas. This method allows the recording of blood flow in the altered areas and using the degree of vascularization to differentiate gastric cancer and other benign changes. Usually in a normal gastric wall it is not possible to visualize the vessels.
Among the 61 patients, pathological vascularization was detected in 42 (68.8%) cases. Of these, all 4 (6.5%) patients with gastric cancer were stage T4 and 38 (62.3%) were stage T3. In 13 cases, vascularization was weak, in 24 cases - moderate, and in 5 cases - enhanced.
Among the 61 patients, pathological vascularization was detected in 42 (68.8%) cases. Of these, all 4 (6.5%) patients with gastric cancer were stage T4 and 38 (62.3%) were stage T3. In 13 cases, vascularization was weak, in 24 cases - moderate, and in 5 cases - enhanced.
Weak vascularization was manifested in the form of a single point color vascular signals, the moderate vascularization - in the form of linear color vascular signals and the enhanced vascularization - in the form of wide color areas inside the tumor (Figure 16, Figure 17).
Figure 16.Distal gastric carcinomas of diffuse infiltrative form of T4 stage. Despite the large size of the tumor the vascularization is manifested in the form of single point color vascular signals.
Figure 17.Gastric carcinomas of infiltrative ulcerative form of Ts stage. Despite the small size of the tumor the vascularization is manifested in the form of wide color areas inside the tumor.
Metastasis to the regional lymph nodes was diagnosed in 52 cases. Ultrasonographically, this was detected in 37 (71.2%) cases. Sonographic features of lymph node metastatic lesions are: an increase in their thickness, a decrease in echogenicity, deterioration or lack of differentiation of the peripheral and central parts of the nodes, recording of color vascular signals inside the nodes (Figure 18, Figure 19).
Figure 18.Metastasis to the retroperitoneal lymph node in gastric cancer of the antrum of T3 stage. The longitudinal size of the lymph node is increased to 5 cm, the echo is significantly reduced, along the periphery of it is visible color vascular signals in tissue Doppler mode.
Figure 19.Metastatic affected lymph node in gastric cancer of the anterior wall of the stomach of stage T4. Significantly enlarged lymph node, echogenicity reduced, large vascular signals in color Doppler mode visible in the center.
Discussion
It is an established fact that, upper gastrointestinal endoscopy and endoscopic ultrasonography are the main methods for diagnosing early gastric cancer 14, 18. Because there are no clinical symptoms for early gastric cancer, these methods can better be utilized in screening programs which is not done in most countries. As a consequence of the gastric cancer screening program in Japan, there is a high rate of early detection 4.
Endophytic growth of gastric cancer for a very long time can remain outside the diagnostic capability of endoscopic and X-ray examination. In such cases, multi-detector computer tomography is the most widely used and effective method 19. In the differentiation of the layers of the stomach wall, especially of its output and body sections, ultrasonography demonstrates a good capability 15.
A computer tomography reconstruction method alternatively, allows for the visualization of vessels in the affected part of the stomach 17. As shown in our study, ultrasonography in tissue mode and color Doppler allows for the visualization of vessels in pathologically thickened areas of the stomach, this can therefore be used to adjudge the intensity and the degree of malignancy of the process.
Diagnosis of lymph nodes involvement in the pathological process allows us to determine the prevalence of gastric cancer and predict survival rate after surgical resection. Double contrast-enhanced ultrasonography is a non-invasive, convenient and reproducible method for the evaluation of LN metastasis in EGC and pre-operative prognosis of EGC 20.
The overall accuracy, sensitivity and specificity of N staging with MDCT are 79% (69–92%), 84.6% (78–92%) and 73.9% (62–85.7%) respectively 21, 22. There is increasing evidence that examination of an insufficient number of lymph nodes may have a detrimental effect on the overall survival rate of patients with GC who receive treatment 23.
In our study, the frequency of visualization of pathologically affected lymph nodes reaches 71%.
Conclusions
In the diagnosis of locally advanced gastric cancer, ultrasonography demonstrates a very good capability for determining the extent and depth of the affected area. In the color Doppler mode, the method allows to study the vascularization of a locally thickened area, as well as adjacent enlarged lymph nodes, which is very important in the determination of the degree of malignancy of the hyperplastic process. Ultrasonography can independently determine the degree of pyloric stenosis in patients with distal gastric cancer.
What this Study Adds
The demonstration of the capabilities of ultrasonography in the diagnosis of gastric cancer
Highlighted the importance of assessing the degree of malignancy of the gastric cancer process and the relation to survival rate.
Study design employed the ultrasound criteria in the diagnosis of EGC which is an effective, efficient and a cheaper imaging method especially in settings with limited health care resources in poor countries.
References
- 1.Field K, Michael M, Leong T.Locally advanced and metastatic gastric cancer: current management and new treatment developments. , Drug 68(3), 299-317.
- 2.International Agencyfor Research on Cancer. [Accessed February25,2013]. GLOBOCAN2008: Stomach cancer incidence, mortality and prevalence worldwide in2008summary. http://globocan.iarc.fr/factsheet.asp.
- 3.Kram A, Peychewa M, Bachurska S, Domagala W. (2011) Morphometric distinction of signet-ring cell adenocarcinoma cells from foamy macrophages in gastric endoscopic biopsies. , Pol J Pathol 62, 145-147.
- 4. (2011) Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. , Gastric Cancer 14, 101-112.
- 5.Ahn H S, Lee H J, Yoo M W. (2011) Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. , Br J Surg 98, 255-260.
- 6.Scaringi S, Kianmanesh R, Sabate J M. (2008) Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 34, 1246-1252.
- 7.Orditura M, F De Vita, Muto P. (2010) Adjuvant chemoradiotherapy in patients with stage III or IV radically resected gastric cancer: a pilot study. , Arch Surg 145, 233-238.
- 8.Soyuer S, Soyuer I, Unal D, Ucar K, Yildiz O G et al. (2010) Prognostic significance of CD9 expression in locally advanced gastric cancer treated with surgery and adjuvant chemoradiotherapy. Pathol Res Pract. 206, 607-610.
- 9.Han S-K.Efficacy of laparoscopic subtotal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (KLASS-0 2-RCT) NCT01456598. VerifiedJuly,2012. http://clinicaltrials.gov/ct2/show/NCT0145659
- 10.A D Sol, Trastulli S, Srassi V.Requirement for a standardised definition of advanced gastric cancer. Oncol Lett.2014Jan. 7(1), 164-170.
- 11.Hallinan J T P D, S K Venkatesh. (2013) Gastric carcinoma: imaging diagnosis, staging and assessment of treatment response. doi: 10.1102/1470-7330.2013.0023.Cancer Imaging.
- 12.Yang D M, Kim H C, Jin W. (2007) 64 multidetector-row computed tomography for preoperative evaluationof gastric cancer: histological correlation. , J Comput Assist Tomogr 31, 98-103.
- 13.Puli S R, J Batapati Krishna Reddy, Bechtold M L, Antillon M R, Ibdah J A. (2008) How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. , World J Gastroenterol 14, 4011.
- 14.Mocellin S, Marchet A, Nitti D. (2011) EUS for the staging of gastric cancer: a meta-analysis. , Gastrointest Endosc 73, 1122-1134.
- 15.R Y Abdullaiev, I V Kryzhanovskaya, Y A Vinnik, Gorleku Ph N. (2018) . Ultrasound Diagnosis in Assessment of Structures and Functional Disorders in Distal Gastric Cancer. Med J Clin Trials Case Stud 2(3), 000138.
- 16.Edge S B, Compton C C. (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. , Ann Surg Oncol 17(6), 1471-1474.
- 17.Moschetta M, Stabile Ianora AA, Anglani A, Marzullo A, Scardapane A et al. (2010) Preoperative T staging of gastric carcinoma obtained by MDCT vessel probe reconstructions and correlations with histological findings. , Eur Radiol.doi;10.1007/s00330-009-1482-7.PMID;19504100 20, 138-145.
- 18.Thrumurthy S G, Chaudry M A, Hochhauser D, Ferrier K, Mughal M Chaudry et al. (2013) The diagnosis and management of gastric cancer". , British Medical Journal 347(16), 1695-6.
- 19.Yan C, Zhu Z G, Yan M. (2009) Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. , J Surg Oncol.Doi;10.1002/jso.21316.Pmid;19530124 100, 205-214.
- 20.Xue N, Huang P, Aronow W S, Wang Z, Nair C K et al. (2011) Predicting lymph node status in patients with early gastric carcinoma using double contrast-enhanced ultrasonography. , Arch Med Sci 7, 457-464.
- 21.Chen C Y, Hsu J S, Wu D C. (2007) Gastric cancer: preoperative local staging with 3D multi-detector row CT–correlation with surgical and histopathologic results. Radiology.Doi;10.1148/radiol.2422051557. Pmid;17255419 242, 472-482.