Ameliorative Potential of Chlorogenic Acid on Rotenone-Induced Neurotoxicity in Drosophila Melanogaster Model
Abstract
Chlorogenic acid (CA), abundantly found in green coffee beans, is a phenolic compound with antioxidant and anti-inflammatory properties amongst others. Exposure to rotenone, a natural pesticide, induces Parkinsonism (a type of neurodegeneration) through the induction of mitochondria dysfunction and oxidative stress. Phytochemicals with antioxidant properties may be promising in attenuating this condition. In this research, the ameliorative role of CA on rotenone-induced toxicity in Drosophila melanogaster was evaluated.
Drosophila melanogaster (Harwich strain, 1- 3 days old) was used. 6 groups of five vials each with 50 flies/vial were exposed to CA (0; control (2% ethanol), 7.5, 15, 30, 45 and 60 mg/kg diet) for 28 days in the longevity analysis. A 28-day survival assay was carried out with rotenone (0, 250 and 500 μM). CA (30 mg/kg diet) was selected to evaluate its ameliorative potential on rotenone. For the study, the flies were divided into four groups of five vials each and exposed to CA and rotenone; Group A- control (2% ethanol), Group B- CA only, Group C- rotenone only and Group D- CA (30 mg/kg diet)+ rotenone (500 μM)for 7 days. Thereafter, the homogenate was evaluated for oxidative stress status, rate of emergence, negative geotaxis and acetyl cholinesterase activity.
CA (30 mg/kg diet) extended the lifespan of flies by 21.4%. Also, CA ameliorated rotenone-induced perturbation in catalase, glutathione-S-transferase and acetyl cholinesterase activities, total thiol and glutathione levels, and behavioral deficit (p < 0.05).
CA may have ameliorative effect against rotenone-induced toxicity and Parkinsonism.
Author Contributions
Academic Editor: Wang Wang, Department of Pediatrics the University of North Carolina at Chapel Hill.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Oluwatoyin Adenike Adeyemo Salami, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Chlorogenic acid (CA) is a polyphenol from the family of hydroxycinnamic acid. It is one of the main polyphenols in the human diet with many health-promoting properties. CA can be found in foods and herbs such as coffee beans, apples, tea, grapes, tomato etc. 1, 2. Furthermore, it has been found that chlorogenic acid has antioxidant, anti-inflammatory, anticancer, antilipidemic, antidiabetic, antihypertensive and anti-neurodegenerative activities 3, 4, 5, 6, 7, 8.
Rotenone (ROT) is a commonly used natural pesticide from the roots of Derris elliptica and Lonchocarpus utilis9. It can cross the blood brain barrier, due to its lipophilic property and causes neurotoxicity 10, 11. ROT blocks the mitochondrial electron transport chain through the inhibition of complex I which causes cytotoxicity due to altered redox cycling and oxidative stress 12. It has been observed that high doses of ROT can induce generalized neurodegeneration, therefore, investigations have been carried out on low-dose systemic administration of this compound in rodents. In this condition, there is slow and specific loss of dopaminergic neurons and impaired mobility in animals treated with ROT, which are attributes of Parkinson’s disease 13.
Physical aberrations characterized by Parkinson’s disease (PD) (a type of neurodegenerative disease) include progressive impairment in mobility ability such as bradykinesia, tremor and rigidity. Studies have shown that PD resulted from the loss of dopaminergic neurons in substantia nigra pars compacta (SNpc) and the formation of intra neuronal proteinaceous inclusions called Lewy bodies (LBs) in affected brain areas 14, 15, 16, 17. Moreover, the pathology involves protein aggregates apart from LBs, various neurotransmitters and extensive regions of the nervous system 18.
In this study, using ROT-induced toxicity in Drosophila melanogaster as a model for neurodegeneration, the ameliorative effect of CA was evaluated.
Materials and Methods
Chemicals
All chemicals were of analytical grade. ROT and CA were procured from AK Scientific, 30023 Ahern Ave, Union City, CA 94587, United State of America.
Drosophila Melanogaster Culture
Drosophila melanogaster (Harwich strain) obtained from the National Species Stock Centre, Bowling Green, Oklahoma, U.S.A., were maintained and reared in the Drosophila Laboratory, Department of Biochemistry, University of Ibadan, Oyo State, Nigeria on cornmeal medium containing brewer's yeast (1% w/v), agar-agar (1% w/v), and nipagin (preservative, 0.08% v/w) at constant temperature (23 ± 2 °C) under 12 h dark/light cycle.
Rotenone and Chlorogenic Acid Exposure and Survival Rate Analyses
In order to determine the appropriate concentrations and duration of exposure to ROT and CA to be used for the main experiment, D. melanogaster (both genders, 1 to 3 days old) were allocated into three (3) groups of five vials with 50 flies each and administered ROT (0- control( 2% ethanol), 250, and 500 µM doses respectively) for 28 days for survival assay while CA was exposed to another six (6) groups of five vials with 50 flies each (0- control( 2% ethanol), 7.5, 15, 30, 45, and 60 mg/kg diet respectively) for 28-day longevity assay. For both assays, daily mortality was recorded, and data were analyzed and plotted as percentage of live flies. The effect on antioxidant status of exposure of six (6) groups of five vials with 50 flies each to CA (0- control( 2% ethanol), 7.5, 15, 30, 45, and 60 mg/kg diet respectively) was determined by assaying in the homogenate for catalase (CAT), glutathione-S-transferase (GST), hydrogen peroxide (H2O2), total thiol and reduced glutathione (GSH) after 7 days. Based on these data, 30 mg/kg diet of CA as well as 500 µM dose of ROT were selected for the study and used to determine the effect on biochemical parameters; acetylcholinesterase (AChE) activity (a marker for neurotoxicity) and oxidative stress-antioxidant status ((H2O2, total thiol, GSH levels, and CAT and GST activities), as well as the rate of emergence of offspring and negative geotaxis.
Determination of Negative Geotaxis and Rate of Emergence of Offspring
Preparation of Sample for Biochemical Assays
For the determination of biochemical assays, 50 flies (of both gender)/ vial for 5 vials each per group, were exposed as described in each of the following groups: Group A (control)-ethanol (2%), Group B- CA only (30 mg/kg diet), Group C- ROT only (500 µM) and Group D- 500 µM ROT + 30 mg/kg diet CA for 7 days. At the end of the treatment period, flies were anaesthetized using CO2, weighed, and homogenized in 0.1 M phosphate buffer, pH 7.0 (ratio of 1 mg:10 ml), and centrifuged at 4000 g for 10 min at 4oC in a Thermo Scientific Sorval Micro 17R centrifuge. Subsequently, the supernatants were separated from the pellets into labeled Eppendorf tubes and used for the determination of the activities of AChE, GST, CAT, and total thiol, H2O2 and GSH content. All the assays were carried out in duplicates for each of the five replicates.
Determination of Biochemical Parameters
Estimation of Protein Concentration
The concentration of protein was evaluated by the method of Lowry et al.22.The sample (25 µl, 1:10 dilution) was mixed with 135 µl of distilled water, and added to 400 µl of alkaline copper sulphate reagent (Lowry reagent) followed by 15 minutes incubation time. Folin ciocalteau solution (1:5 dilution) was added and they were then incubated at room temperature for 20 mins. The absorbance was measured at a wavelength of 660 nm against blank and protein values were estimated from the standard curve of bovine serum albumin (BSA).
Estimation of Total Thiol Level
The method of Ellman 23 was used to determine total thiol content. The reaction mixture consists of 510 μl of 0.1 M phosphate buffer (pH 7.4), 20 μl of sample, 35 μl of 1 mM 5′,5′ – dithiobis- 2- nitrobenzoate (DTNB) and 35 μl of distilled water. The mixture was incubated for 30 mins at room temperature and the absorbance was measured at 412 nm.
Estimation of Hydrogen Peroxide Level
The level of H2O2 in the treatment was determined using the method of Wolff 24. The reaction mixture consist of FOX 1 (10 ml of 100 mM xylenol orange, 50 ml of 250 mM ammonium ferrous sulfate, 10 ml of 100 mM sorbitol, 5 ml of 25 mM H2SO4 and 30 ml of distilled water) mixed with the sample. Incubation was carried out for 30 mins at room temperature and the absorbance was measured at 560 nm. The values, which were deduced from the standard curve, were expressed in micromole per milligram protein.
Estimation of Reduced Glutathione Level
The estimation of the level of GSH was carried out with the method of Jollow et al.25 with little modifications. Sulphosalicyclic acid (4%) in the ratio of 1:1 was used to precipitate the supernatant. The samples were incubated for 1 h at 4oC and then centrifuged at 5000 rpm for 10 mins at 4oC. The reaction mixture consists of 550 µl of 0.1 M phosphate buffer, 100 µl of supernatant and 100 µl of DTNB. The absorbance was read at 412 nm and the results were expressed as moles of GSH/gram tissue.
Estimation of Glutathione-S-Transferase Activity
The method of Habig and Jakoby 26 was used to assess the activity of GST. The reaction mixture comprised of 270 μl of a solution consisting of 20 μl of 0.25 M potassium phosphate buffer, pH 7.0, with 2.5 mM ethylene diamine tetra acetic acid (EDTA), 10.5 ml of distilled water and 500 μl of 0.1 M GSH at 25 °C),10 μl of 25 mM 1- chloro-2,4- dinitrobenzene (CDNB; as substrate) and 20 μl of sample (1:5 dilution). The mixture was monitored for 5 min with 10 s intervals at 340 nm using a spectrophotometer.
Determination of Catalase Activity
CAT activity was assessed with the method of Aebi 27. The reaction mixture consists of 50 mM of phosphate buffer (pH 7.0), 300 mM H2O2 and sample (1:50 dilution). The loss in absorbance of H2O2 was monitored for 2 min at 240 nm. The result was used to calculate CAT activity and expressed as μmol of H2O2 consumed per minutes per milligram of protein.
Determination of Acetyl Cholinesterase Activity
AChE activity was evaluated using the method of Ellman et al.28. The reaction mixture contained 135 μl of distilled water, 20 μl of 100 mM potassium phosphate buffer (pH 7.4), 20 μl of 10 mM DTNB, 5 μl of sample, and 20 μl of acetylthiocholine (8 mM). The reaction was monitored at a wavelength of 412 nm (for 5 min, 15 s intervals) in a SpectraMax microplate reader (Molecular Devices, USA). The data were expressed thereafter in μmol/min/mg protein.
Statistical Analysis
All of the experiments were replicated at least two times. The data are presented as the mean ± standard error of mean (SEM). To assess the significant differences among multiple groups under various treatments, One-way analysis of variance (ANOVA) was used. For all of the assays, differences with p-value < 0.05 were considered statistically significant.
Results
Longevity and Selected Biochemical Indices in Flies Exposed to Chlorogenic Acid
Life time exposure to CA (7.5, 30, 45 and 60 mg/kg diet) increased longevity of flies by 3.57%, 21.4%, 14.3% and 7.1% respectively while there was a reduction (-3.57% ) at the 15 mg/kg diet (Figure 1). In addition, CA maintained CAT (Figure 2A) and GST (Figure 2B) activities in most of the groups, and reduced H2O2 levels in the 15, 30 and 60 mg/kg diet (Figure 2C) after exposure to flies for 7 days when compared with the control flies (p < 0.05). CA (30, 45, and 60 mg/kg diet) concentrations maintained total thiol level in flies when compared with the control (Figure 2D). Moreover, 45 mg/kg diet of CA significantly increased GSH (Figure 2E) levels in flies when compared with the control group (p < 0.05). Generally, 30 mg/kg diet of CA prolonged longevity and maintained redox balance of the flies significantly (p < 0.05) when compared with the other groups and the control flies. Therefore, 30 mg/kg concentrations was selected to investigate the ameliorative role on ROT-induced toxicity in the flies.
Figure 1.Influence of chlorogenic acid on longevity
Effects of Rotenone on Survival of Drosophila Melanogaster
Figure 3 shows the effects of ROT on survival of D. melanogaster after 28 days of treatment with 250 µM and 500 µM of rotenone concentrations. The group treated with 500 µM of ROT concentration shows an increase mortality rate when compared with the control (p < 0.05). Hence 500 µM concentration was chosen for the study.
Figure 3.Influence of rotenone on survival of D. melanogaster after 28 days of treatment
Effects of Chlorogenic Acid on the Redox State of Rotenone Exposed Flies
Figure 4A shows the effects of CA and ROT on the H2O2 level in Drosophila melanogaster after 7 days of treatment. There was no significant effect upon administration of CA on the ROT-treated group when compared with control (p < 0.05). There was a significant elevation in CAT activity in the ROT-treated group which was ameliorated by CA (Figure 4B; p < 0.05). In addition, Figure 4C shows the levels of total thiol and GSH (Figure 4D), and GST activity (Figure 4E) in D. melanogaster exposed to ROT and CA. CA significantly mitigated ROT- induced decrease (p < 0.05) in the levels of total thiol and GSH when compared with the control (p < 0.05). Also, CA significantly increased ROT-induced inhibition of GST activity to levels comparable with the control (p < 0.05). Comparing the CA treated ROT group with the ROT only group, administration of CA significantly (p < 0.05) reduced CAT activity (Figure 4B) and significantly increased total thiol level (p < 0.05) (Figure 4C).
Figure 4.Effect of chlorogenic acid on rotenone induced alterations on oxidative stress markers.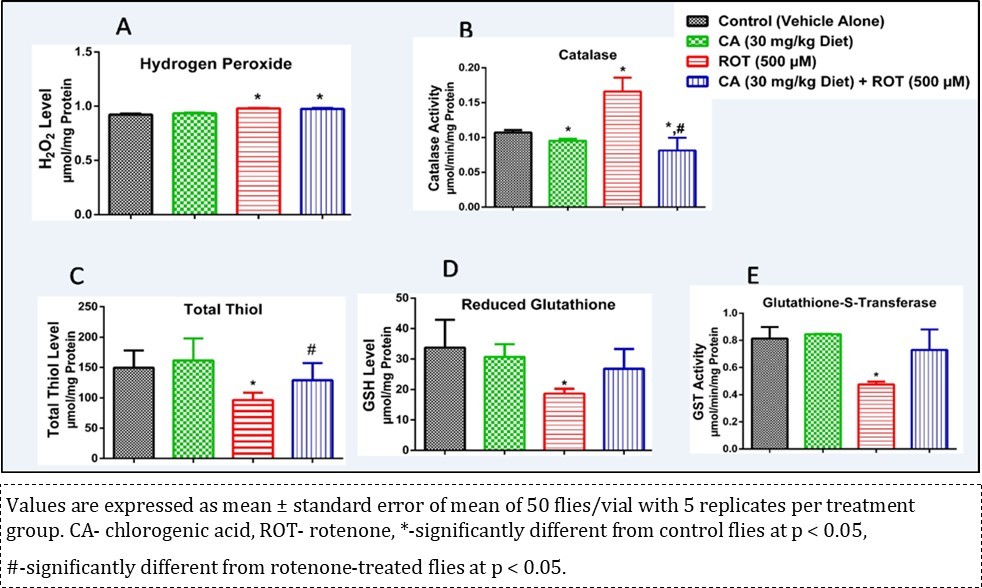
Acetyl Cholinesterase and Behavioural Assays
A significant reduction in the climbing rate of flies was observed (Figure 5A, p < 0.05) in ROT-treated flies after 7 days when compared with the control. However, CA reversed ROT-induced behavioural deficit depicted by the climbing rate (p < 0.05). In addition, ROT had a significant adverse effect on the fertility of flies, however CA alleviated ROT-induced alteration in fertility depicted by eclosion rate after 7 days of treatment (Figure 5B, p < 0.05). CA also significantly (p < 0.05) alleviated ROT-induced inhibition of AChE activity to levels comparable to control (Figure 5C). Comparing the CA treated ROT group with the ROT only group, administration of CA significantly (p < 0.05) increased the climbing and eclosion rates, and the AChE activity.
Figure 5.Influence of chlorogenic acid on negative geo taxis, flies’ emergence and acetyl cholinesterase activity in rotenone-treated flies.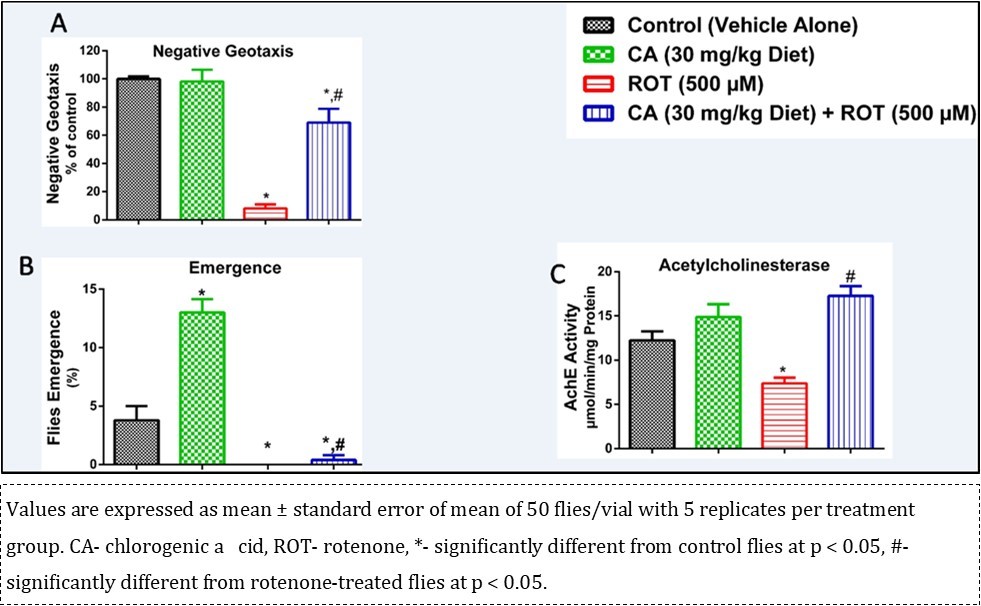
Discussion
In this study, it was demonstrated in Drosophila melanogaster that CA ameliorated the deleterious effects of ROT on AChE and GST activities, total thiol and GSH levels, and climbing and eclosion rates.
PD is an age-related neurodegenerative disease affecting more than 1% of the human population. It is the second most common neurodegenerative disorder with prevalence increasing with age, mostly among population above 60 years 29. The symptoms of PD include progressive impairment in locomotive ability, tremor, rigidity, and bradykinesia 17. Currently, a combination of genetic changes and environmental factors has been observed to be responsible for this condition, though controversially 30, 31. Some of the environmental factors is the exposure to chemicals in pesticides and herbicides such as ROT, permethrin, MPTP (1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine), organochlorines, paraquat and 2, 4-dichlorophenoxyacetic acid 32, 33, 34. In this study, ROT was used to induce Parkinsonism in Harwich strain of Drosophila melanogaster.
Currently, the modulatory role of several nutraceuticals to attenuate endogenous oxidative stress status has been considered as an effective approach to achieve neuroprotection 35, 36, 37. As stated earlier, CA is a phenolic compound found in food of plant origin which has anti-diabetic, antioxidant, anti-inflammatory, anticancer, antilipidemic, and anti-neurodegenerative properties 3, 4, 5, 6, 7, 8. Moreover, studies have shown that consumption of diets rich in antioxidant property contribute to extension of lifespan in an organism 38. The fly-survival and longevity experiment based on CA showed that among the concentration range tested (7.5- 60 mg/kg diet), 30 mg/kg of CA was the most effective (Figure 1). Therefore, in regard to the ingestion of CA, moderate levels would be more beneficial to increase longevity. Moreover, CA significantly reduced the level of H2O2 with the 30 mg/kg diet and did not show any adverse effect on the activities of CAT and GST as well as the levels of total thiol and GSH (Figure 2).
In this study, it was observed that 28-day exposure of D. melanogaster to ROT elicit alterations in the levels of antioxidant enzymes activities (such as CAT and GST), perturbations in reduced total thiol and GSH levels, increased H2O2 level, induced behavioural deficit as well as decreased AChE activity. All the aforementioned detrimental effects observed (with the exception of H2O2 level) were mitigated by CA (Figures 4 and 5).
CAT catalyzes the conversion of H2O2 to water (H2O) and oxygen. The most common one is a monofunctional heme-containing enzyme 39. It is an intracellular antioxidant enzyme that protects biological macromolecules from oxidation by H2O239. This study shows an elevated CAT activity in flies exposed to ROT which was mitigated to levels comparable to control by CA (Figure 4B). This reveals the modulatory role of CA in obtaining an optimum level of H2O2 molecule in the cell, which is also essential in cellular signaling and this is supported by the observation with the CA diets (Figure 2C).
H2O2 is a reactive oxygen species (ROS). Elevated levels of H2O2 indicate that a system is plagued by oxidative stress 39. In this study it was observed that there was reduction in the level of H2O2 in flies treated with CA alone. However, ROT caused a significant accumulation of H2O2 which was mitigated, but not significantly, by CA (Figure 4A). Antioxidants play protective roles by scavenging reactive oxygen species and reducing the insults of oxidative damage 40. Although CA did not significantly reduce H2O2 level in the co-administered flies, the reduction in CAT activity upon co-administration shows that CA has an ameliorative effect on ROT-induced toxicity but was not distinct enough on H2O2 level upon conclusion of the experiment.
Total thiols (protein and non-protein thiols) defend the system against oxidative damage 41. GSH functions as part of the first line of antioxidant defense system in living organisms. In conjunction with GSH-dependent enzymes, it helps to block the propagation of free radical chain reactions and detoxify deleterious ROS 42. GSH is an important antioxidant in living organisms that prevents oxidative damage to cellular components 42, 43, 44. Depletion of cytosolic GSH in neurons is one of the hallmarks in PD 45. The decrease in levels of total thiols and GSH in the ROT challenged group evidenced from our study suggests a compromised defense system in D. melanogaster. However, this study shows that CA modulates ROT-induced reduction of thiol containing compounds, thus confirming its anti-oxidative capacity. Hence, CA may be an ameliorative agent against diseases associated with altered redox state of a system.
GST is a major phase II detoxification enzyme, which comprises of a large and multi-functional enzyme family primarily, involved in the detoxification of endogenous substrate by catalyzing the conjugation of the nucleophilic sulphur atom of GSH with a variety of reactive electrophilic exogenous compounds 34, 46, 47. The result from our study shows that CA protected against ROT-induced inhibition of GST activity. Since GST catalyses the conjugation of GSH with electrophilic molecules, GSH was rapidly used up in order to protect the flies. Therefore, the ameliorative mechanism of CA in ROT-induced toxicity may be by further enhancing activity of thiol-containing proteins like GST.
AChE is a vital enzyme of the cholinergic system that modulates learning, sleep and wakefulness, memory, attention, stress response and locomotor activities. It hydrolyzes acetylcholine to choline and acetate thereby terminating cholinergic neurotransmission between synapses 48, 49. Our study revealed that CA significantly alleviated ROT-induced inhibition of AChE activity when compared with control. This finding suggests that the modulatory action of CA on AChE activity may improve cognitive impairment evident in neuro-related diseases.
In the negative geotaxis assay, it was observed that ROT decreased the climbing rate of the flies but the effect was mitigated in groups co-treated with CA. This shows that CA has an ameliorative potency against bradykinesia, which is one of the effects of ROT toxicity and a hallmark in PD. This is similar to the study conducted by Farombi et al. 21, in which kolaviron was able to modulate the effect of ROT in flies.
Flies’ emergence was highly increased in the group treated with CA only but a barren effect was recorded in the group treated with ROT only as compared with the control group. Also, substantial flies’ emergence was observed in the co-administered group treated. This shows that CA may have a highly ameliorative effect against reproduction deficit common in PD patients. This is in line with the study conducted by Namula et al. 50 in which CA, in addition with caffeic acid, was able to improve the quality of frozen-thawed boar sperm 50.
Conclusion
The present study demonstrates that chlorogenic acid showed substantial ameliorative potential on rotenone-induced Parkinsonism in Drosophila melanogaster as shown by the reversal of the detrimental effects of rotenone on the levels and activities of total thiol, reduced glutathione, glutathione-S-transferase and acetylcholinesterase. The negative geotaxis and developmental assays further buttressed the ameliorative effect of chlorogenic acid. These findings indicate that chlorogenic acid might have therapeutic effects for Parkinson’s disease. Hence, majorly consumed home diet should be fortified with chlorogenic acid or there should be an adequate intake of foods rich in chlorogenic acid to prevent or modulate the effect of rotenone exposure in our environment as well as delay or possibly halt the onset of neurodegenerative diseases such as Parkinsonism.
Data Availability Statement (DAS)
The data is available upon request from the Corresponding author.
Abbreviations
CA- chlorogenic acid, ROT- rotenone, PD- Parkinson’s disease, SNpc- substantia nigra pars compacta, LBs- Lewy bodies, AChE- acetylcholinesterase, DTNB- 5′,5′ – dithiobis- 2- nitrobenzoate, GST- glutathione - S - transferase, CAT-catalase, CDNB-1- chloro-2,4-dinitrobenzene, EDTA- ethylene diamine tetra acetic acid, GSH- reduced glutathione, ROS- reactive oxygen species, MPTP- 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine.
References
- 1.Niggeweg R, Michael A J, Martin C. (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. , Nat. Biotechnol 22, 746-54.
- 2.Bhattacharyya S, Majhi S, Saha B P, Mukherjee P K. (2014) Chlorogenic acid- phospholipid complex improve protection against UVA induced oxidative stress. , J Photochem Photobiol B 130, 293-8.
- 3.Yagasaki K, Miura Y, Okauchi R, Furuse T. (2000) Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. , Cytotechnology 33, 229-35.
- 4.Noratto G, Porter W, Byrne D, Cisneros-Zevallos L. (2009) Identifying peach and plum polyphenols with chemo preventive potential against estrogen-independent breast cancer cells. J Agric Food Chem. 57, 5219-26.
- 5.Lee B, Choi H Y, Jeong C H, Bu Y. (2012) Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. , Eur J Pharmacol 689, 89-95.
- 6.Hwang S J, Kim Y W, Park Y, Lee H J, Kim K W. (2014) Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW264.7 cells. Inflamm Res. 63, 81-90.
- 7.Nabavi S F, Tejada S, Setzar W N, Gortzi O, Sureda A et al. (2017) Chlorogenic acid and mental diseases: From Chemistry to Medicine. , Curr Neuropharmacol 15, 471-9.
- 8.Yan Y, Zhou X, Guo K, Zhou F, Yang H. (2020) Use of chlorogenic acid against Diabetes mellitus and its complications. , J Immunol Res 9680508.
- 10.Santiago R M, Barbieiro J, Lima M M, Dombrowski P A, Andreatini R et al. (2010) Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson's disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry. 34, 1104-14.
- 11.Swarnkar S, Singh S, Mathur R, Patro I, Nath C. (2010) A study to correlate rotenone induced biochemical changes and cerebral damage in brain areas with neuromuscular coordination in rats. , Toxicology 272, 17-22.
- 12.Sherer T B, Betarbet R, Testa C M, Seo B B, Richardson J R et al. (2003) Mechanism of toxicity in rotenone models of Parkinson's disease. , J Neurosci 23, 10756-64.
- 13.Fleming S M, Zhu C, Fernagut P, Mehta A, DiCarlo C D et al. (2004) Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. , Exp. Neurol 187, 418-29.
- 14.L S Forno. (1996) Neuropathology of Parkinson's disease. , J Neuropathol Exp Neurol 55, 259-72.
- 15.Nussbaum R L, Polymeropoulos M H. (1997) Genetics of Parkinson's disease. Hum Mol Genet. 6, 1687-91.
- 17.Jankovic J.Parkinson's disease: clinical features and diagnosis. , J Neurol Neurosurg Psychiatry.2008; 79, 368-76.
- 20.Abolaji A O, Kamdem J P, Lugokenski T H, Farombi E O, Souza D O et al. (2015) Ovotoxicants 4-vinylcyclohexene 1,2-monoepoxide and 4- vinylcyclohexene diepoxide disrupt redox status and modify different electrophile sensitive target enzymes and genes inDrosophila melanogaster. Redox Biol. 5, 328-39.
- 21.Farombi E O, Abolaji A O, Farombi T H, Oropo A S, Owa O A et al.(2018).Garcinia kolaseed flavonoid fraction (Kolaviron), increases longevity and attenuates rotenone-induced toxicity inDrosophila melanogaster. Pestic Biochem Phys. 145, 39-45.
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. (1951) Protein measurement with the Folin phenol reagent. , J. Biol. Chem 193, 265-75.
- 24.Wolff S P. (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. , Methods Enzymol 233, 182-9.
- 25.Jollow D J, Mitchell J R, Zampaglione N, Gillette J R. (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 11, 151-69.
- 26.Habig W H, Jakoby W B. (1981) Assays for differentiation of glutathione-S-transferases. Methods Enzymol. 77, 398-405.
- 28.Ellman G L, Courtney K D, Andres V, Feathers-Stone R M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. , Biochem Pharmacol 7, 88-95.
- 29.Reeve A, Simcox E, Turnbull D. (2014) Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor. , Ageing Res Rev 14, 19-30.
- 30.Inamdar N N, Arulmozhi D K, Tandon A, Bodhankar S L. (2007) Parkinson’s disease: Genetics and beyond. Curr Neuropharmacol. 5, 99-113.
- 31.Ball N, Teo W P, Chandra S, Chapman J. (2019) Parkinson’s disease and the environment. Front Neurol. Available from:https://doi.org/10.3389/ fneur.2019.00218
- 32.Brown T P, Rumsby P C, Capleton A C, Rushton L, Levy L S. (2006) Pesticides and Parkinson’s disease- Is there a link?. Environ Health Perspect. 114, 156-64.
- 33.Tanner C M, Kamel F, Ross G W, Hoppin J A, Goldman S M et al. (2011) Rotenone, paraquat and Parkinson’s disease. Environ Health Perspect. 119, 866-72.
- 34.Abolaji A O, Adedara A O, Adie M A, Vicente-Crespo M, Farombi E O. (2018) Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1,2,3,6- tetra hydropyridine-induced oxidative damage and behavioural deficits inDrosophilamelanogaster. , Biochem Biophys Res Commun 503, 1042-1048.
- 35.Jimenez-Del-Rio M, Guzman-Martinez C, Velez-Pardo C. (2010) The effects of polyphenols on survival and locomotor activity inDrosophila melanogasterexposed to iron and paraquat. Neurochem Res. 35, 227-38.
- 36.Dumont M, Beal M F. (2011) Neuroprotective strategies involving ROS in Alzheimer Disease. Free Radic Biol Med. 51, 1014-26.
- 37.Prasad S N, Muralidhara. (2012) Evidence of acrylamide induced oxidative stress and neurotoxicity inDrosophila melanogaster- its amelioration with spice active enrichment: relevance to neuropathy. Neurotoxicology. 33, 1254-64.
- 38.Kumar A, Christian P K, Panchal K, Guruprasad B R, Tiwari A K. (2017) Supplementation of Spirulina (Arthrospira platensis) improves lifespan and locomotor activity in Paraquat-Sensitive DJ-1βΔ93flies, a Parkinson's Disease model inDrosophila melanogaster. J Diet Suppl. 14, 573-88.
- 39.Nandi A, Yan L J, Jana C K. (2019) Role of catalase in oxidative stress- and Age-associated degenerative diseases Oxid Med Cell Longev. 9613090.
- 40.Kurutas E B. (2016) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. , Nutr J 15, 71.
- 41.Ulrich K, Jakob U. (2019) The role of thiols in antioxidant systems. , Free Radic Biol Med 140, 14-27.
- 42.Lushchak. (2012) Glutathione homeostasis and functions: Potential targets for medical interventions. J Amino acids. 736837.
- 43.Sharma P, Jha A B, Dubey R S, Pessarakli M. (2012) Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. , J Bot 2012, 217037.
- 44.Biller J D, Takahashi L S. (2018) Oxidative stress and fish immune system: phagocytosis and leukocyte activity burst. , An. Acad. Bras. Ciênc
- 45.Garrido M, Tereshchenko Y, Zhevtsova Z, Taschenberger G, Bahr M et al. (2011) Glutathione depletion and overproduction both initiate degeneration of nigral dopaminergic neurons. , Acta Neuropathol 121, 475-85.
- 46.Mathew N, Kalyanasundaram M, Balaraman K. (2006) Glutathione-S- Transferase (GST) inhibitors. Expert Opin Ther Pat. 16, 431-44.
- 47.Suthar P C. (2017) Glutathione-S-Transferases: A brief on classification and GSTM1-T1 activity. IJPSR. 8, 1023-7.
- 48.Ferreira-Vieira T H, Guimaraes I M, Silva F R, Ribeiro F M. (2016) Alzheimer’s disease: Targeting the cholinergic system. , Curr Neuropharmacol 14, 101-15.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
Basic & Clinical Pharmacology & Toxicology (2025) OpenAlex
Basic & Clinical Pharmacology & Toxicology (2025) Crossref

