Transmutation of Sweat Glands - Eccrine Porocarcinoma
Abstract
Initially described by Pinkus and Mehregan in 1963 as an epidermotropic eccrine carcinoma, eccrine porocarcinoma cogitates an exceptional sweat gland malignancy. Eccrine porocarcinoma was adapted as a nomenclature by Mishisma and Morikoin in 1969. The neoplasm is a malignant analogue of eccrine poroma which is a benign tumour of intra-dermal sweat glands. Eccrine porocarcinoma is an invasive malignancy of eccrine sweat gland with an acrosyringial genesis. Nomenclature includes epidermotropic eccrine carcinoma, eccrine poroepithelioma, malignant hidroacanthoma simplex, malignant intra-epidermal eccrine poroma, malignant eccrine poroma, malignant syringoacanthoma and dysplastic poroma (1,2). Sweat gland carcinoma are categorized into subgroups with the classical eccrine porocarcinoma or eccrine adenocarcinoma as a prevalent subcategory. Lesions are enlisted as
Classic type eccrine adenocarcinoma ( eccrine porocarcinoma).
Syringoid eccrine carcinoma
Microcystic adnexal carcinoma
Mucinous eccrine carcinoma
Muco-epidermoid carcinoma
Adenoid cystic carcinoma
Aggressive digital papillary adenoma/adenocarcinoma
Author Contributions
Academic Editor: Qiping Dong, MD. (Pathology) Panjab University, Department of Histopathology, A.B. iagnostics, A-1, Ring Road, Rajouri Garden, New Delhi, 110027, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Anubha Bajaj
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Preface Eccrine porocarcinoma is designated as an exceptional sweat gland malignancy which was initially described by Pinkus and Mehregan in 1963 as an epidermotropic eccrine carcinoma. Subsequently, Mishisma and Morikoin in 1969 adopted the nomenclature of “eccrine porocarcinoma”. The neoplasm is a malignant analogue of eccrine poroma which is a frequently elucidated benign tumour of intra-dermal sweat glands. Eccrine porocarcinoma is an invasive malignancy of eccrine sweat glands and demonstrates an acrosyringial genesis. Additionally, the neoplasm is designated as epidermotropic eccrine carcinoma, eccrine poroepithelioma, malignant hidroacanthoma simplex, malignant intra-epidermal eccrine poroma, malignant eccrine poroma, malignant syringoacanthoma and dysplastic poroma 1, 2. Sweat gland carcinomas are categorized into subgroups along with the classical eccrine porocarcinoma or eccrine adenocarcinoma as a prevalent subcategory. Lesions are enlisted as
Classic type eccrine adenocarcinoma (eccrine porocarcinoma).
Syringoid eccrine carcinoma
Microcystic adnexal carcinoma
Mucinous eccrine carcinoma
Muco-epidermoid carcinoma
Adenoid cystic carcinoma
Aggressive digital papillary adenoma/adenocarcinoma2, 3
Disease Characteristics
Eccrine porocarcinoma is described as an infrequent malignant adnexal neoplasm engendered from intra-epidermal portion of sweat glands. The tumour has an estimated prevalence of 0.005% to 0.01% of epithelial cutaneous neoplasm. Eccrine porocarcinoma commonly occurs in the elderly within 6th and 7th decades and manifests a female predominance or an equivalent gender predisposition. Duration of lesions can vary from 2 months to 50 years. Porocarcinoma frequently appears on the lower extremity (>50%), trunk (24%), head (18%) and rarely in regions delineating a congregation of eccrine sweat glands. Torso, head and neck, abdomen, upper limbs, scalp, breast, vulva, scrotum and nail bed are infrequently implicated. Lesions of the scalp are discerned in beneath < 20% instances 2, 3. Eccrine porocarcinoma can emerge as a de novo disorder or sequential to a preceding lesion such as eccrine poroma, nevus sebaceous, chronic lymphocytic leukaemia or actinic keratosis. An estimated 20% eccrine poromas delineate a malignant transformation 2, 3.
Eccrine porocarcinoma can be concurrent to immune suppression (transplant recipients, HIV infection, chemotherapy), radiation exposure and chronic radiation dermatitis. Eccrine porocarcinoma can arise in association with extra-mammary Paget’s disease, sarcoidosis, pernicious anaemia, Hodgkin’s lymphoma, HIV infection or xeroderma pigmentosum 3, 4. Abrupt metamorphosis of a benign neoplasm into a nodular, infiltrative, ulcerated or polypoid tumefaction is indicative of malignant conversion. The exceptional eccrine porocarcinoma exhibits a variable clinical appearance with an absence of distinctive features. Thus, a definitive clinical diagnosis necessitates an adequate histological concordance. Lesions can be misdiagnosed as squamous cell carcinoma, basal cell carcinoma, seborrheic keratosis or metastatic adenocarcinoma.
Extensive tissue sampling of the tumefaction exhibits an infiltrative pattern of tumour evolution with nuclear enlargement and mitotic activity. Cytological atypia and stromal infiltration can classify the lesion as a porocarcinoma 2, 4.
Expeditious tumour progression, ulcerative manifestation and multiple lesions indicate a localized reoccurrence or metastasis to adjacent viscera such as lymph nodes, lungs, liver, urinary bladder, peritoneum or retro-peritoneum 4, 5.
Clinical Elucidation
An ulcerative plaque, nodule or a tumefaction is exemplified clinically. Frequently, a reddish, nodular, cauliflower like excrescence or verrucous plaques with infiltration of adjacent tissue and an ulcerated, haemorrhagic superimposed epidermis is discerned. Enlarged, painless, enhancing, exophytic, reddish papules or tumefaction can also be cogitated. Lesions can be present for long duration with a sudden augmentation in magnitude and serous discharge. Tumours can range from < 1 centimetre to 10 centimetres in magnitude.
A non healing, nodular, haemorrhagic, ulcerated, polypoidal lesion with an inverted perimeter and magnitude of 8 centimetres to 10 centimetres can be cogitated. A loco-regional tumour infiltration is discerned with recent and rapid progression 4, 5.
The neoplasm is devoid of regional lymph node enlargement. Surgical borders can be devoid of / or may exhibit tumour infiltration. Absence of specific clinical attributes and appearance of benign poroid features can engender a diagnostic dilemma 5, 6.
Histological Elucidation
Malignant transformation into an eccrine porocarcinoma from an adjacent poroma can be discerned on histology. Thus, tissue specimens can demonstrate a cytological uniformity, appear devoid of anaplasia and depict a benign composition. Tumour perimeter can indicate the emergence of an infiltrating squamous cell carcinoma or carcinoma of skin adnexa with squamous differentiation.
Eccrine porocarcinoma is an infiltrative, high grade tumour which is contiguous with superimposed epidermis, depicts a partially lobular architecture and a diameter usually exceeding > 90 millimetres. An endophytic pattern of tumour evolution is elucidated with invasion of deep reticular dermis and subcutaneous tissue 5, 6.
Eccrine porocarcinoma on morphological elucidation depicts the incrimination of cutaneous structures, foci of ulceration along with multiple areas of cellular infiltration. Tumefaction arises from basal layer of skin.
Classically, intra-epidermal and dermal nests of tumour cells exhibiting cellular atypia and enhanced mitotic activity are enunciated. Tumour aggregates articulate well demarcated, enlarged, atypical polygonal cells with indistinct cellular and nuclear outline, nuclear hyperchromasia , irregular nuclei, vesicular or prominent nucleoli and minimal eosinophilic cytoplasm. Polygonal tumour cells can depict central keratinisation 6, 7.
The neoplasm is composed of lobules of aberrant epithelial cells configured in cords with incrimination of the dermis and epidermis.. Malignant cells congregate within the epidermis or infiltrate the dermis, especially in the primary tumour. Tumour cells within tumour aggregates display a well demarcated cellular outline and appear distinct from encompassing squamous cells. Numerous tumour cell clusters demonstrate a cystic lumen. Prominent epidermal acanthosis is discerned on account of tumour cell proliferation 6, 7.
Granular arrangement of malignant cells and intercellular bridges are conspicuous with the demonstration of nuclear atypia, pleomorphism, prominent mitosis and tumour necrosis. A peripheral palisade is discernible within the cellular aggregates. Mitotic figures are common and can be quantified as up to 12 mitosis/ high power field. Tumour differentiation can prominently be of the ductal category with the demonstration of intra-cytoplasmic lumina. Comedo type tumour necrosis is evident along with foci of squamous differentiation The neoplasm is reactive to periodic acid Schiff ‘s (PAS) stain. (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13.
Figure 1.Solid aggregates of tumour cells and duct structures in eccrine porocarcinoma (14).
Figure 2.Epidermal projections lined with atypical and malignant epithelial cells in eccrine porocarcinoma (14).
Figure 3.Cohesive accumulations with numerous ductular articulations of carcinoma cells in eccrine porocarcinoma (15).
Figure 4.Cellular atypia, mitosis and focal necrosis in eccrine porocarcinoma(16).
Figure 5.Sweat glandular articulations and malignant cellular aggregates with cystic spaces in eccrine porocarcinoma(17).
Figure 6.Epidermal continuity with aggregates of atypical, solid and cystic epithelial cell nests in eccrine porocarcinoma (18).
Figure 7.Aberrant and malignant epithelium with cellular proliferation and pleomorphism in eccrine porocarcinoma with frequent mitosis(19).
Figure 8.Disseminated atypical epithelial cells with cellular and nuclear pleomorphism, hyperchromasia, indistinct cytoplasm, vesicular nucleoli and central keratinization in eccrine porocarcinoma(20).
Figure 9.Branches and cords of malignant epithelial cells in eccrine porocarcinoma with a superficial, abutting epithelium and mitotic figures(21).
Figure 10.Duct like configurations and atypical epithelial cells with prominent mitosis in eccrine porocarcinoma (22).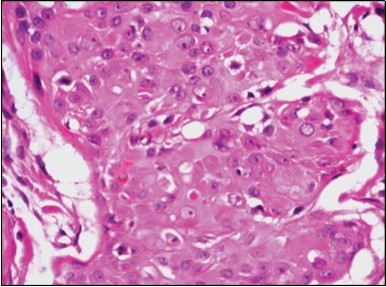
Figure 11.Eccrine porocarcinoma displaying cords and strands of atypical epithelial cells with glandular arrangements (23).
Figure 12.Immune reactivity to cyto-keratin (CK7) in eccrine porocarcinoma(24).
Figure 13.Immune reactivity to CK6 in eccrine porocarcinoma(25).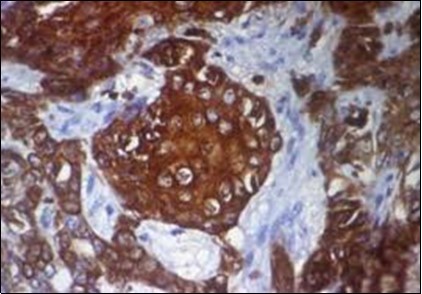
Following proliferation and epidermal infiltration, the tumefaction can invade papillary and reticular dermis. Implication of dermal lymphatic vessels ensures tumour dissemination to regional lymph nodes (20%) or isolated solid organs (10%) or demonstrable localized tumour reoccurrence(20%) 2, 4.
Eccrine porocarcinoma manifests lymph node invasion with capsular disruption and focal infiltration of surgical margins. Nodules upon the cutaneous surfaces delineate metastasis within the dermis and subcutaneous fat with extensive lymphatic and vascular permeation 7, 8.
Histological subtypes of eccrine porocarcinoma include
Pushing variant –polypoid lesions with a well defined inferior tumour margin.
Infiltrative variant –Indistinct inferior border of the neoplasm with tumour cell aggregates infiltrating the dermis and/or hypodermis.
Pagetoid variant- Intra-epidermal dissemination of tumour cells besides the delineation of primary neoplasm - recapitulating Paget’s disease.
Tumour nodules can appear horizontally within the epidermis with peripheral extension, invasion of underlying dermis or subcutaneous adipose tissue along with metastasis to internal organs and viscera 7, 8.
Immune Histochemical Evaluation
Eccrine porocarcinoma is immune reactive to carcino-embryonic antigen (CEA) and cyto-keratins 6 and 7(CK6,7), epithelial membrane antigen (EMA) and non reactive to S-100 protein. Reactivity to cyto-keratin 20 (CK20) assists the diagnosis in instances with indeterminate histology. Immune reactive p63, CK5 , weak P40 and non reactive GATA3 are additionally elucidated by the tumour 2, 3, 4.
Differential Diagnosis
Nodules of eccrine porocarcinoma located on extremities necessitate a segregation from entities such as seborrheic keratosis, pyogenic granuloma, amelanotic melanoma, squamous cell carcinoma, basal cell carcinoma or verruca vulgaris.
Tumour cell aggregates infiltrating the epidermis require a demarcation from conditions such as hidroacanthoma simplex, Bowen’s disease or seborrheic keratosis 7, 8.
Scalp lesions require a differentiation from conditions such as cylindroma, eccrine poroma, sebaceous adenoma, sebaceous carcinoma, pilar tumour and metastatic carcinoma.
Additionally, a distinction is required from eccrine poroma, hidradenoma, hidroadenocarcinoma, squamous cell carcinoma and basal cell carcinoma in instances with extensive dermal infiltration 8, 9.
Eccrine porocarcinoma necessitates a differentiation from conditions such as
Porocarcinoma
Basal Cell Carcinoma
Seborrheic Keratosis (clinical differentiation)
Pyogenic granuloma (clinical differentiation)
Acrochordon (clinical differentiation)
Amelanotic melanoma (clinical differentiation)
Verrucae
Hidradenoma
Hidroadenocarcinoma
Bowen’s disease (squamous cell carcinoma in situ)
Paget’s disease
Squamous cell carcinoma
Metastasis (pulmonary carcinoma, adjuvant malignancy)
Infiltrating duct carcinoma breast
With specimens obtained from superficial and shave biopsies, it can be challenging to differentiate eccrine poroma from non invasive (in situ) eccrine porocarcinoma or pushing variant of eccrine porocarcinoma. However, eccrine poroma lacks attributes such as dermal invasion, marked cytological atypia and necrosis 8, 9.
Eccrine porocarcinoma can be differentiated from infiltrating basal cell carcinoma due to the demonstration of intercellular bridges, absence of peripheral palisade and tumour retraction artefact 2, 3.
Squamoid eccrine porocarcinoma can be distinguished from squamous cell carcinoma which lacks duct-like articulations and intra-cytoplasmic duct lumina. Aforesaid features can be discerned with adequate immune reactivity to EMA and CEA in eccrine porocarcinoma.
Infiltrating primary duct carcinoma of the breast necessitates a distinction from eccrine porocarcinoma appearing within breast tissue or axillary lymph nodes. Misdiagnosis can occur in an estimated 30% instances.
Eccrine porocarcinoma depicts contiguity of the tumour to superficial epidermis with ductal eccrine differentiation. Cogent immune reactions with non reactive cyto-keratin (CK7), hormone receptors (oestrogen or progesterone) or GATA3 enunciates an absence of primary breast carcinoma 3, 4.
Eccrine porocarcinoma is diffusely immune reactive to p63 and cyto-keratin 5 (CK5), in contrast to a metastatic carcinoma of the skin which is non reactive p63 or CK5 9, 10.
Investigative Assay
A comprehensive medical history with incidents of exposure to toxins and carcinogens is mandated. Extensive evaluation of the implicated skin and adjacent lymph nodes is required. Ultrasound can be performed for analysing enlarged lymph nodes. Fine needle aspiration cytology can depict a primary or metastatic carcinoma consistent with eccrine porocarcinoma. Computerized tomography of the pelvis, abdomen and thorax can be performed and exhibits a poorly demarcated, variable, dense, soft tissue lesion devoid of erosion of the underlying bone 9, 10.
Computerized tomography can also disclose an enlarged tumour mass with infiltration of adjoining soft tissue and incriminated regional lymph nodes. Fluoro-deoxy glucose (FDG PET CT) positron emission computerized tomography of localized tumour aggregates depict intense FDG avid tumour nodules or infiltrated lymph nodes.
FDG PET CT scans employed for elucidating a reoccurrence in porocarcinoma can display multiple FDG avid regions on the cutaneous surface and regional lymph nodes. Repetitive FDG PET CT scans can expose additional lesions confined to the skin, bone, lungs or lymph nodes 9, 10.
Primary tumour and subsequent metastasis exhibits an enhanced glucose metabolism which aids appropriate detection 10, 11.
Positron emission tomography ( PET CT) can be utilized to similarly delineate tumour nodules or incriminated lymph nodes. However, a close monitoring is advised as PET CT demonstrates a reduced sensitivity to miniature lesions. Comprehensive bone scan can be ordered to detect adjuvant lesions or exclude bone metastasis. Magnetic resonance imaging is employed to indicate additional locations of the neoplasm. Magnetic resonance imaging delineates the target tumour volume with identification of adjoining neural / vascular structures 10, 11.
Prognostic Determinants
Attributes of eccrine porocarcinoma associated with inferior prognosis include mitotic activity and tumour thickness besides lymphatic and vascular invasion. Neoplasm displaying an invasive tumour border instead of a pushing inferior tumour margin is associated with enhanced percentage of tumour reoccurrence.
Tumour reappearance occurs in an estimated one third (35%) instances and is commonly elucidated with pagetoid or infiltrative histological subcategories. Pushing subtype seldom relapses. Emergence of infiltrative tumour perimeter appears to be predictive of localized tumour reoccurrence 4, 5.
Extent of tumour resection margin (≥2 centimetres) or (< 2 centimetres) or adoption of narrow resection margin with modified Moh’s micrographic surgery (MMS) may not influence prognostic outcomes, irrespective of the histological subtype encountered. Inferior prognostic outcome with frequent mortality is indicated with aspects such as mitotic figures beyond > 14 mitosis /high power field, lymphatic and vascular invasion with lymph node metastasis, depth of tumour invasion exceeding > 7millimteres and infiltrative tumour perimeter 2, 4.
Multi-nodular or ulcerative, rapidly enhancing lesions can reoccur subsequent to adoption of a wide surgical excision. Consequently, an unfavourable prognosis and mortality ensues due to malignant metastasis. Aforesaid instances are unresponsive to chemotherapy or radiotherapy. A mortality rate of up to 80% can be encountered in invasive, ulcerated or multi-nodular tumours 9, 10.
Therapeutic Options
Benign poroma necessitates a therapeutic surgical extermination as an estimated 20% instances can transform into infiltrative, malignant neoplasm. Simple surgical excision ensures a complete alleviation of the condition for miniature lesions. Alternatively, Moh’s micrographic surgery can be employed for enlarged or deep-seated lesions. Superficial lesions can be managed by a fulguration , cautery, surgical elimination or electro-cauterization.
Non metastatic eccrine porocarcinoma is appropriately alleviated by a surgical eradication with a broad tumour perimeter 11, 12.
Adoption of surgical excision with a wide margin in early eccrine porocarcinoma enunciates a proportionate cure rate of 70% of 80%. Comprehensive surgical resection of the tumour is a preferred treatment modality. Surgical extermination with removal of a tumour perimeter of one centimetre is advocated. Skin grafting can be employed to repair the surgical defect 12, 13.
Singular surgical elimination is curative in a majority (70%) instances. Aggressive, localized surgical eradication with a tumour free surgical perimeter is optimal. Infiltrated tumour margins also mandate a wide surgical extermination on account of extensive regional metastasis.
Surgical dissection can be challenging on account of intense adherence of the tumefaction to circumscribing soft tissue and vascular configurations. Complications such as post operative haemorrhage and infection mandate reparative intervention. Suitable surgical elimination of the primary lesion exemplifies a proportionate alleviation of beyond > 80% with a reoccurrence of beneath < 20% 11, 13.
Moh’s micrographic surgery also provides a cogent therapeutic option. Minimally aggressive pushing subtype of eccrine porocarcinoma can be managed with surgical resection of a narrow margin of uninvolved tissue. Modified Moh’s micrographic surgery can be adapted for eliminating infiltrative or pagetoid subtype of eccrine porocarcinoma 3, 4.
Regional lymph nodes require evaluation as porocarcinoma depicts a predilection for invasion of dermal lymphatic vessels with a consequent tumour burden within the regional lymph nodes in an estimated 20% instances. Incriminated regional lymph nodes necessitate an appropriately expansive lymph node dissection. Prophylactic and extensive dissection of regional lymph nodes is advocated in approximately 20% instances with inferior prognostic outcomes which are characteristically delineated by lymphatic or vascular invasion, infiltrating or pagetoid tumour variant or an enhanced grade of lesions cogitated with localized tumour reoccurrence 8, 9.
Adjuvant radiation therapy is an additional modality which is recommended in subjects with infiltration of surgical perimeter. Unfavourable prognostic parameters such as focal infiltration of resection margin, prominent lymphatic and vascular invasion, lymph node incrimination and capsular disruption can mandate the employment of adjuvant radiotherapy.
Chemotherapy is a cogent modality for managing metastatic lesions. Eccrine porocarcinoma depicts loco-regional tumour reappearance in an estimated 20% subjects with visceral or bone metastasis in approximately 10% instances following adequate surgical eradication of primary tumefaction 12, 13.
Nevertheless, surgical re-excision remains the therapeutic paradigm in locally or regionally metastatic eccrine porocarcinoma. Comprehensive radiotherapy and chemotherapy can also be considered as an option.
Individual survival can be enhanced in metastatic disease with the employment of palliative chemotherapy and/or radiotherapy. Systemic chemotherapy with tamoxifen or docetaxel is an efficacious alternative to surgery 12, 13.
References
- 1.Pinkus H, Mehregan A H. (1963) Epidermotropic eccrine carcinoma: a case combining features of eccrine poroma and Paget’s dermatosis”. , Arch Dermatol 88, 597-606.
- 2.Monten C, Berwouts J. (2018) A case of eccrine porocarcinoma located in the breast – the pitfalls reviewed “ iMedPub Journals –Medical Case Reports. 4(1), 004-18.
- 3.Inamdar B, Naik R. (2018) Eccrine porocarcinoma of scalp : an unusual malignancy of the sweat glands” Clinic in Surgery. 3, 1865.
- 4.Seo B F, Choi H J. (2018) Eccrine porocarcinoma on the cheek”Archives of Craniofacial Surgery. 20(1), 48-50.
- 7.Chang O, Elnawani A. (2011) Eccrine porocarcinoma of the lower extremity : a case report and review of literature”. , World J Surg Oncol 9, 94.
- 8.Golemi A, Hanspeter E. (2014) FDG/PET CT in malignant eccrine porocarcinoma arising in a pre-existing poroma”. , Clin Nuc Med: 39, 456-458.
- 9.Skowron F, Poulhalon N. (2014) Primary eccrine porocarcinoma : a clinicopathological study of 50 cases”. , Ann Dermatol Venereol: 141, 258-264.
- 10.Reira Leal L, Guevara-Gutierrez. (2015) Eccrine porocarcinoma: epidemiologic and histopathologic characteristics”. , Int J Dermatol 54, 580-586.
- 11.Aaribi I, Mohataram A. (2013) Successful management of eccrine porocarcinoma “ Case Rep Oncol Med:. 282536.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
Journal of Neurosurgery Case Lessons (2021) OpenAlex
Journal of Neurosurgery: Case Lessons (2021) Crossref
Journal of Clinical and Diagnostic Pathology (2019) OpenAlex
