Feasibility of Detecting Brain Areas Involved in Extreme Breath-Hold Diving
Abstract
A feasibility study explores neuroimaging approaches to identify brain regions engaged during extreme breath‑hold diving. Methods, safety considerations, and preliminary activation patterns are summarized to motivate further controlled studies.
Author Contributions
Academic Editor: Sasho Stoleski, Institute of Occupational Health of R. Macedonia, WHO CC and Ga2len CC, Macedonia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Patrice Jissendi-Tchofo, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Breath-hold diving (BH-diving) triggers a complex adapting mechanism called “diving response” that is the result of several psycho-physiological components 1. The major physiological components of the diving response that occur during BH-diving are peripheral vasoconstriction, bradycardia and decreased cardiac output, on the other hand we have an increase of cerebral and myocardial blood flow, an increase of blood pressure and splenic constriction that ensure adequate oxygen delivery to the brain and to the heart 2, 3. The increase of CO2 level (hypercapnia) contributes to dyspnea sensation, which also leads to others physiological responses and adaptation mechanisms 4. Some authors focused on the significant increase of cerebral blood flow (CBF) in elite divers, as compared to non-Breath Hold divers (BH-divers), and showed that even if hypoxia and hypercapnia occur at the end of long BH-diving, some oxygen-conserving mechanisms can occur 5. Cardiovascular magnetic resonance imaging (CMR), performed to investigate changes in the cardiovascular system during BH-diving, showed that prolonged BH-diving caused stress in the cardiovascular system, however with no sign of acute myocardial injury 6. The effect of prolonged BH-diving on the brain is poorly understood field of investigation. During BH-diving, the brain is rapidly subject to an increase of the hypoxia, which is responsible for the loss of consciousness that can occur at the end of a prolonged BH-diving 7.
Cerebral Decompression illness (DCI) mechanism in BH-divers is still controversial and many possible pathogenic mechanisms have been considered as caused of neurological symptoms in BH-diving 8, a recent case report described high bubble formation, recorded by echocardiography, in a BH-diver consolidating the hypothesis that, at least in some cases, bubbles formation could be involved in cerebral injures 9.
Finally brain MRI with fluid-attenuated inversion recovery (FLAIR) has been used to evaluate the lesions of two breath-hold divers 10.
Recent developments in MRI techniques allow today the functional brain mapping using venous blood oxygenation level-dependent (BOLD) MRI which relies on changes in deoxyhemoglobin, with a higher sensitivity at high magnetic fields 11. This in-depth technique that permits a functional MRI (fMRI) has been introduced to evaluate brain activity by detecting changes associated with the blood flow 12.
The fMRI technique has developed in different variants that can be classified into two main types: resting state (RS-FMRI) versus action/event-related fMRI 13. RS-fMRI aims at recording the BOLD signals all over the brain while the patient is at rest. Then, robust statistical analyses enable to detect concordant areas of the brain that resonate at low frequencies, resulting in network nodes for areas that resonate at similar frequencies 14. Despite all this knowledge, there aren’t data about fMRI applications to underwater activities. The aim of our study was to detect the concordant functioning areas of the brain during prolonged breath-holds in a world-class apnea diver, by means of RS-fMRI.
Materials and Methods
Subject and Breath-Hold Protocol
The study was conducted on a 40-year-old male, elite diver (world record holder), with high experience in breath holding. The subject signed an informed consent form prior to the study, which was approved by the Academic Bioethical Committee (Haute Ecole Paul Henri Spaak, Brussels, Belgium), according to the declaration of Helsinki. He also expressively gave his permission for the publication of the identifiable data presented. Subject was relaxed because he had participated in prior MRI studies in which no abnormalities had been found 15.
At the time of MRI, subject was wearing light personal clothes. He was in a dorsal decubitus position with arms along the body and kept eyes closed during the entire experiment. Breath-hold was not preceded by glossopharyngeal breathing (lung “packing”) or prolonged hyperpnoea. He was free to start and stop the apnoea and the experiment at any moment while was in the magnet.
The subject performed two different maximal breath holds. Both protocols lasted 12 min 45 sec. There were only a few minutes delay between both breath holding tests.
First breath holding (FBH): (5.44 min.)
FBH0; From 0 to 220 s (scan 44): normal breathing
FBH1; From 221 to 547 s (scan 109): breath hold
FBH2; From 548 to 765 s (scan 150): normal breathing
Second breath holding (SBH): (5.77 min.)
SBH; From 0 to 240 s (scan 48): normal breathing
SBH; From 241 to 587 s (scan 117): breath-hold
SBH; From 588 – 765 s (scan 150): normal breath
Equipment and Data Acquisition
The start/stop breath-holding time-points were recorded upon an alarm “bip” using a sucker connected to the magnet in the subject’s right hand. The experiment was performed in a 3 Tesla Magnet (Achieva R3 Philips, Best, The Netherlands) with a SENSE-Head-8 channels coil. The peripheral arterial oxygen (HbO2) saturation and heartrate (HR) were monitored and recorded using a finger probe, MR 3T compatible (MEDRAD MR Monitoring System VERIS, Model 8600). The ambiance in the magnet room was dim light.
The anatomical images were acquired with a FFE (fast field echo) sagittal 3DT1 1 mm isotropic voxel sequence (TR/TE: 9.7/4.6 ms; flip angle 8; FOV 256 mm; matrix 256x256; 160 slices). The experiments were performed subsequently using a gradient echo EPI (echo planar imaging) sequence (TE: 2500/40 ms, TR: 5000 ms, FOV: 300, matrix 128x127, voxel size AP/RL/FH: 2.34/2.34/4.00 mm, EPI: 75, 150 dynamic scans), covering the entire brain with 35 slices in a total scan time of 12 min 45s (765s) for each.
Data Processing
fMRI data was preprocessed using the statistical parametric mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB2010a. Images were co-registered and realigned using a least square approach and 6 parameters rigid body spatial transformation in order to reduce motion artifacts. The realigned images were therefore segmented and stereotactically normalized into the Tailarach space 16. Finally, images were smoothed using a Gaussian kernel with a Full Width at Half Maximum (FWHM) at 6 mm in the 3 dimensions.
Next, the data was processed using the Group Independent Component Analysis (ICA) of fMRI Toolbox (GIFT) (http://mialab.mrn.org/software/gift/index.html) and Matlab. The number of independent components (ICs) was estimated using the MDL criteria 17. The 150 BOLD time-points (corresponding to 150 dynamic scans) underwent decorrelation analyses and then principal component analysis (PCA) decomposition. Finally, the most significant ICs were extracted using the Fast ICA algorithm (http://research.ics.aalto.fi/ica/fastica/). Temporal components of all data sets were sorted using kurtosis criteria and only the activation maps with highest kurtosis values were considered. Activated areas were mapped on T1 anatomical images, and rendered as multiplanar images. The time course (TC) of BOLD signals of the ICs was plotted as the average curve within the activated area along with the minimum and the maximum curves.
Results
We found the dorsal pons, cerebellar hemispheres (superior aspect) and whole vermis, bilateral dorsal and ventromedial prefrontal, and medial occipital cortices to show significant BOLD signals differences as compared to the whole brain, during the first breath holding (Figure 1).
Figure 1.First experiment showing both BOLD signal time courses and activated areas: dorsal pons (1st row), anterior and posterior vermis as well as superior and lateral aspects of the cerebellar hemispheres (2nd row), dorsomedial prefrontal cortex (3rd row), and primary visual cortex (last row).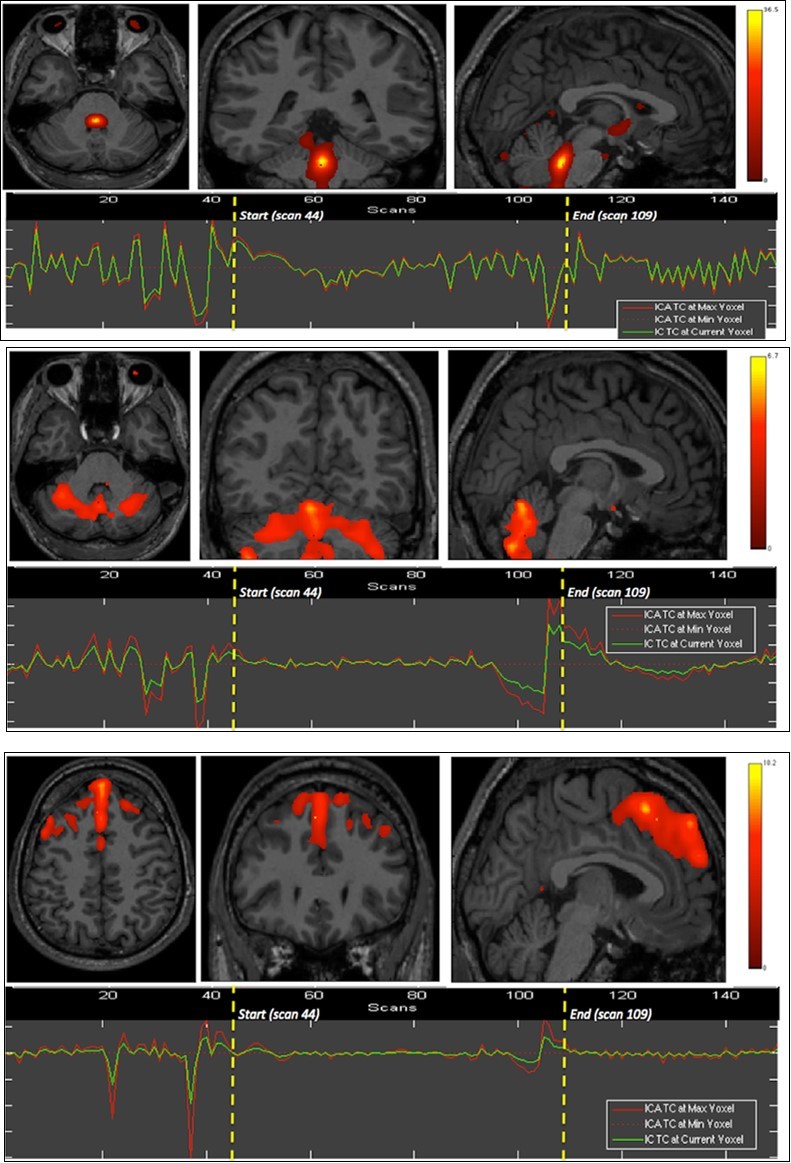
Right insula, bilateral orbitofrontal and right inferior parietal cortices were eloquent during the second breath holding (Figure 2).
Figure 2.Second experiment showing BOLD time courses and activation of ventromedial prefrontal cortex (upper row) and inferior parietal cortex including the angular gyrus (middle row) and the supramarginal gyrus (lower row).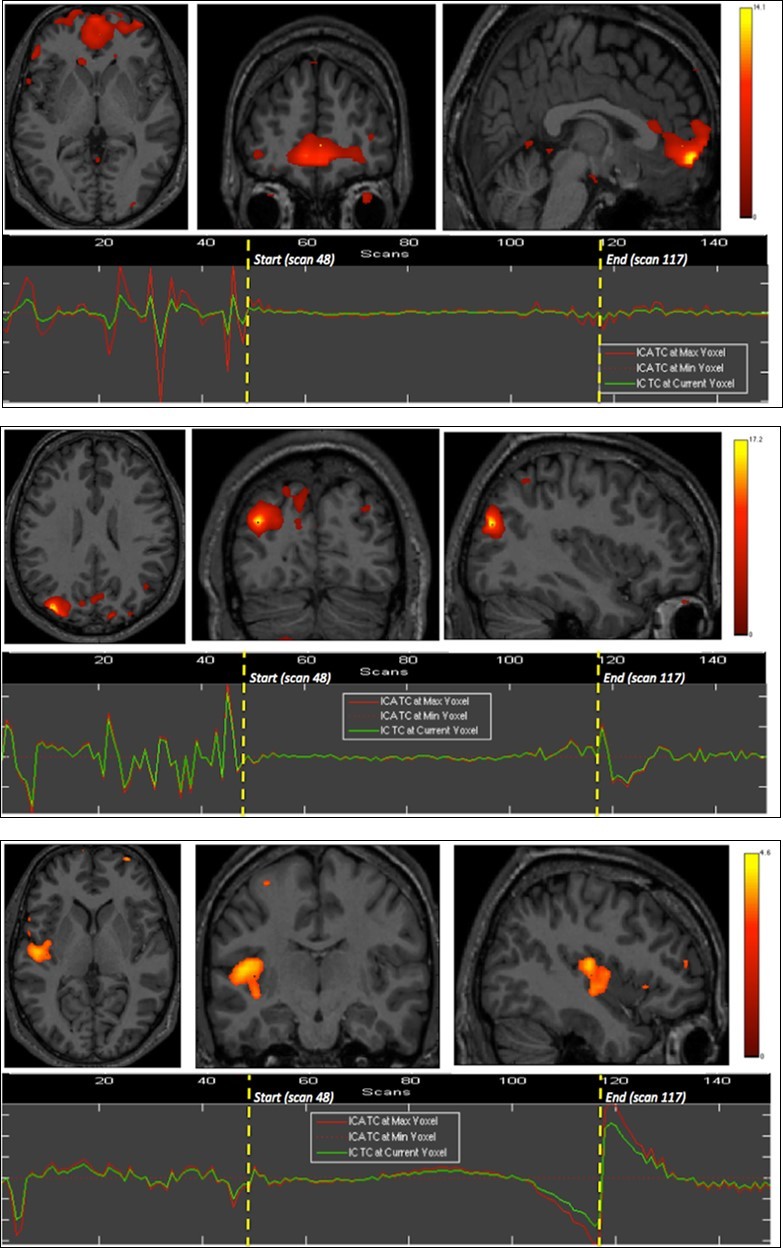
The apnea period was obviously recognizable on the time course of the BOLD signals graphs because of the striking change of the curves from breathing to apnea and after apnea, with very low fluctuations during apnea. Within eloquent brain areas TC curves showed various patterns: (i) high fluctuations mainly in the second half of apnea in the dorsal pons only (Figure 1-1st row), (ii) very low fluctuations with deep depression at the end of apnea in the cerebellum (Figure 1-2nd row) as well as in the supramarginal gyrus (Figure 2, lower row), less marked in occipital areas (figure 1-4th row), and (iii) flat curve in dorsomedial prefrontal (Figure 1-3), ventromedial prefrontal (Figure 2-upper row) and inferior parietal areas (Figure 2-middle row). The eloquent areas disclosed during both experiments are mapped on a brain mesh as nodes of both networks (Figure 3).
Figure 3.Projections of activated areas on a brain mesh showing networks involved in the first apnea (blue labels) and second apnea (red labels). Dorsomedial (dmPFC) and ventromedial prefrontal cortices (vmPFC), dorsal pons (DP), vermis (V) and cerebellar hemispheres (CH) are activated bilaterally, while inferior parietal cortex, including the angular gyrus (AG) and the supramarginal gyrus (SMG), is on the right hemisphere and medial visual cortex (V1) on the left one. R=right. L=left.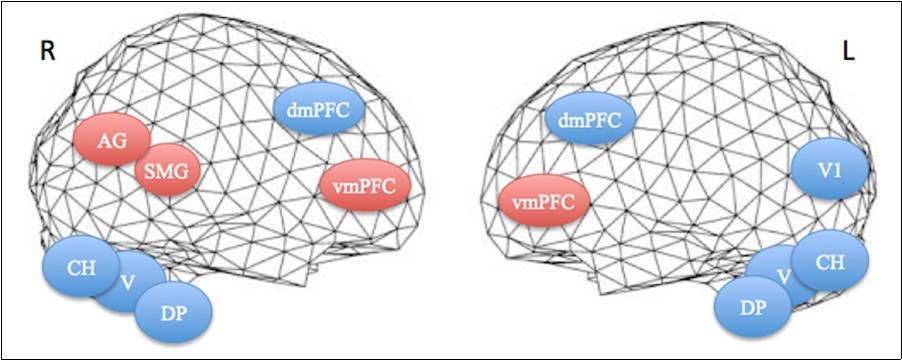
In BH-diving (as in our experiment) the extended time without breathing exposed the subject to brain hypoxia/ hypercapnia associated with a decrease of cardiac output and peripheral HbO2. The Figure 4 show the hypoxia and hypercapnia BH-diving related obtained the same day of fRNM test but during a performance even longer (9 min, 7 sec) which illustrates this aspect.
Figure 4.Cardiac Frequency and Oxygen Saturation (finger probe) during a 547 sec. (9 min 7 sec) dry voluntary breath-hold (n=1)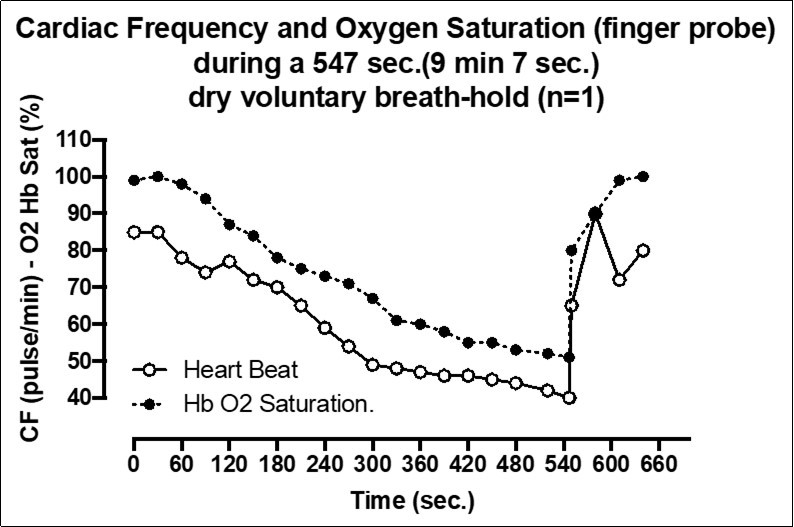
Discussion
To our knowledge, our study is the first to investigate BOLD signals changes in the brain under voluntary static dry long breath-hold. The first challenge in this study was the self-control start/stop apnea of diver. Indeed, as in wet conditions, the subject was free to start and stop breath-holding, and the scan time was set to 765 s in order to record enough scans during the breath-hold period.
Our main findings encompass the brain eloquent areas likely involved to the first and second apnea, namely the brainstem and cerebellum, prefrontal, parietal, occipital and insular cortices. We also found a typical pattern of BOLD signals variation, consistent all apnea long, with some variations during apnea depending upon the eloquent regions of the brain. We assume that the eloquent areas were activated in response to apnea as well as in the control of apnea.
In the brain, variations of oxygenation at the arterial venous junction generate BOLD signals. The signal changes observed are closely related to the changes in arterial oxygen saturation during hypoxia 18. In such conditions, brain oxygenation is preserved by compensatory mechanisms called the “diving response” and brain arterial autoregulation 2, 19.
This response includes systemic changes, notably vagal reaction with bradycardia, peripheral vasoconstriction related to sympathetic nervous system stimulation and spleen contraction, to reduce tissue oxygen uptake, as well as brain vasodilatation, increased cerebral blood flow and fluctuations of the hemoglobin concentration 20. These mechanisms, including both vascular and metabolic changes 21, are presumably responsible for the BOLD signals recorded in our subject.
Independent component analysis (ICA) is one of the most popular methods proven efficient, consistent and reliable, to identify low-frequency resting-state patterns and to show temporal and spatial correlations in the brain 22, 23. We used this method to extract brain areas showing similar neurovascular changes, and thus putatively involved in the same network during apnea. We analyzed separately the two experiments in order to depict the difference between first and recurrent apneas. Indeed, networks disclosed were not identical.
We found the dorsal pons (DP), the vermis (V), the cerebellar hemispheres (CH), and the dorsomedial prefrontal (dmPFC) areas to be correlated in the same network, in the first breath hold, and ventromedial prefrontal cortex (vmPFC), angular (AG) and supramarginal gyri (SMG) of the inferior parietal lobe, in the second breath hold. The DP activation might be directly linked to the reticular formation involved in the sympathetic nervous control, likely elicited by urgent breath requirement associated with the first phase of prolonged apnea. The neurochemical control of breath generation, rhythm and hold, is a complex and not yet completely understood field 24. DP nuclei are part of the complex respiratory system including other brainstem nuclei that are sensitive to carbon dioxide levels and acidity, which concentration depends on blood flow and oxygenation. The brainstem interacts with the cortex for breath regulation via the cortico-pontine and reticulo-spinal tracts 24. The functional mapping of the cerebellar hemispheres has shown that lateral lobules VI and Crus I are involved in motor preparation, while upper medial lobules IV and V are involved in motor execution 25. Crus I is connected with the prefrontal cortex and lobules IV, V and VI with the motor cortex 25, 26. These areas (VI and Crus I) are also involved in verbal working memory, a function that could be part of apnea sustaining. In this topological cerebellar organization, the anterior vermis (lobules I-VI) was proven strongly correlated with the motor and somatosensory cortices, and the posterior vermis (lobules VII-IX) with prefrontal (vm- and dmPFC) and inferior parietal (AG and SMG) cortices. These cerebellar activations could be related to the training effect and emotion processes 25. In addition, the cerebrum cortical areas disclosed have interesting implications in active and resting state networks: PFC for decision procedure, awareness, mentalizing capabilities and resting state default mode network as well as for sustaining activity in working memory and monitoring the response selection. Medial occipital activation may implicate the visual stimulation for perceptual awareness and consciousness. The network we observed is likely responsible for sustaining consciousness and decision making ability, including connections with the respiratory regulator system, via a self-representation or out-of-body (OBE) experience 27.
Ultimately, about the differences in eloquent areas between the first and the second apnea, we can speculate on a “conditioning” effect of the first apnea which could cause a change in subsequent apneas with less neurovascular and neurochemical changes. In the second Breath hold vmP, FC and inferior parietal lobules took the control of apnoea, they are usually involved in decision procedure, awareness, mentalizing capabilities and resting state default mode network as well as for sustaining activity in working memory and monitoring the response selection. In our case, the PFC activation is likely related to the decision making of sustaining apnea although the subject declared that, during the apneas, he was relaxed, “emptying his mind” while imagining a breath-hold dive.
We also observed various patterns of time courses of BOLD signal within activated areas that could correspond to various locoregional neurobiological behaviors during apnea. It’s known that voluntary breath holding is a hypoxia model consisting of two phases of oxygen saturation dynamics: an initial slow decrease (normoxic phase) followed by a rapid drop (hypoxic phase), during which transitory neurological symptoms as well as slight impairment of integrated cerebral functions, such as emotional processing, can occur 28. The decision of stopping the breath-hold might be based on these neurological disturbances. Our patient described an out of the body experience and a “Samba” feeling (myoclonic agitation, presenting usually as agonist/antagonist muscular activities recalling cerebellar activation) during the second apnea. These movements are considered to be likely due to cerebral hypoxia; in our data, metabolites build up is more prone to explain this feeling, happening just before he decided to stop the apnea. This seems to be in line with some experiences reported during altered states of consciousness such as recovery after narcotic states or presyncopal situations 29, 30. Also, it is difficult to comment on the significance of the lateralization of activated areas. We found visual activation in the left hemisphere, inferior parietal and supramarginal gyri in the right hemisphere, while other activation areas were observed bilaterally. This lateralization might be related to the dominant hemisphere, which was likely the right in our left-handed subject.
Our results are limited to one individual. Moreover, they are likely incomplete due to several technical considerations. This was the very first experiment in a challenging situation and the design of the study as well as the data analysis methods will benefit from the present findings to a larger study. We cannot yet hypothesize on the functional circuitry existing between these areas in the induction and sustaining of apnea and the way these nodes interact in the networks.
A recent paper investigated cerebral metabolism and vascular reactivity during breath holds measuring global cerebral blood flow (CBF), metabolic rate of oxygen (CMRO2), and magnetic resonance spectroscopy (MRS) to evaluate the cerebral lactate, glutamate/glutamine, N-acetylaspartate and phosphocreatine/creatine concentrations in the occipital lobe.
We conclude that under hypoxic conditions, the mechanism for sustaining brain function in response to/control of long breath-holding likely involves different areas of the central nervous system (the cerebrum, the brainstem and the cerebellum) implicated in a complex network. Still, more studies are needed to establish a specific relationship between those areas and dry voluntary long breath hold. Our data may stimulate the use of fMRI to better understand brain adaptations strategies during breath-hold diving.
Acknowledgements
We dedicate this paper to Patrick Musimu, world class breath-hold diver who passed away after contributing to this study.
References
- 2.Dujic Z, Breskovic T, Ljubkovic M, Breathholddiving. (2011) in vivo model of the brain survival response in man?Med Hypotheses. 76(5), 737-40.
- 3.Heusser K. (2009) et al.,Cardiovascular regulation during apnea in elite divers.Hypertension. 53(4), 719-24.
- 4.G E Foster.Sheel AW. (2005).The human diving response, its function, and its control.Scand. , J Med Sci Sports 15(1), 3-12.
- 5.C K Willie. (2015) et al.,Regulation of brain blood flow and oxygen delivery in elite breath-hold divers.J Cereb Blood Flow Metab. 35(1), 66-73.
- 6.Eichhorn L. (2018) et al.,Cardiovascular magnetic resonance assessment of acute cardiovascular effects of voluntaryapnoeain elite divers.J Cardiovasc Magn Reson. 20(1), 40.
- 7.Lindholm P.(2007).Loss of motor control and/or loss of consciousness during breath-hold competitions.Int. , J Sports Med 28(4), 295-9.
- 8.G E Foster. (2016) Commentaries on Viewpoint: Why predominantly neurological DCS in breath-hold divers?. , J Appl Physiol 120(12), 1478-82.
- 9.Cialoni D.(2016).Detection of venous gas emboli after repetitive breath-hold dives: case report.Undersea Hyperb Med. 43(4), 449-455.
- 10.Yasuno F. (2014) et al.,Decision-making deficit of a patient with axonal damage after traumatic brain injury.Brain Cogn. 84(1), 63-8.
- 11.Norris D G.(2006).Principles of magnetic resonance assessment of brain function.J. , Magn Reson Imaging 23(6), 794-807.
- 12.S A Huettel, McCarthy S A W.. G.,Functional Magnetic Resonance Imaging. 3 ed2014 , Sunderland, MA (USA) .
- 13.Thompson G J.(2018).Neural and metabolic basis of dynamic resting state fMRI.Neuroimage. 180(Pt B):. 448-462.
- 14.Kim J. (2014) Frequency-dependent relationship between resting-state functional magnetic resonance imaging signal power and head motion is localized within distributed association networks. , Brain Connect 4(1), 30-9.
- 15.Germonpre P, Balestra C, Musimu P. (2011) Passive flooding of paranasal sinuses and middle ears as a method of equalisation in extreme breath-hold diving. , Br J Sports Med 45(8), 657-9.
- 16.Tailarach J T P, Co-Planar.Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - An Approach to Cerebral Imaging. 1 ed1988. , New York (USA)
- 17.O L Li, Adali T, Calhoun.V.,Sample Dependence CorrectionForOrder Selection In FMRI Analysis. in3rd IEEE International Symposium on Biomedical Imaging: Nano to Macro2006, IEEE: , Arlington, VA, USA 1073-1075.
- 18.Rostrup E. (1995) et al.,Signal changes in gradient echo images of human brain induced by hypo- and hyperoxia.NMR Biomed. 8(1), 41-7.
- 19.D R Pendergast. (2015) et al.,Human Physiology in an Aquatic Environment.Compr Physiol. 5(4), 1705-50.
- 20.Breskovic T. (2010) Peripheral chemoreflex sensitivity and sympathetic nerve activity are normal in apnea divers during training season. , Auton Neurosci.154(1-2): 42-7.
- 21.Fukunaga M. (2008) et al.,Metabolic origin of BOLD signal fluctuations in the absence of stimuli.J Cereb Blood Flow Metab. 28(7), 1377-87.
- 22.Margulies D S. (2010) Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity.MAGMA.23(5-6):. 289-307.
- 23.Heuvel MP van den, HE. (2010) Hulshoff Pol,Exploring the brain network: a review on resting-state fMRI functional connectivity.Eur Neuropsychopharmacol. 20(8), 519-34.
- 25.Stoodley C J, Schmahmann J D. (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. , Cortex 46(7), 831-44.
- 26.J X O'Reilly. (2010) Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. , Cereb Cortex 20(4), 953-65.
- 27.Jackson D A. (1995) Out-of-body experience in a patient emerging from anesthesia. , J Post Anesth Nurs 10(1), 27-8.
- 28.Menicucci D. (2014) et al.,Brain responses to emotional stimuli during breath holding and hypoxia: an approach based on the independent component analysis.Brain Topogr. 27(6), 771-85.
Cited by (1)
This article has been cited by 1 scholarly work according to:
Citing Articles:
Brain Structure and Function (2021) OpenAlex
