Abstract
Redox enzymes are a type of enzyme that catalyzes redox reactions, that is, electron transfer reactions between two chemical species. Redox enzymes are essential for many biological processes, including cellular respiration, photosynthesis, energy production, and the elimination of free radicals.
They are divided into two main types: oxidoreductases and electron transferases. Oxidoreductases catalyze the direct transfer of electrons between two chemical species, while electron transferases catalyze electron transfer by cofactors.
Examples of redox enzymes include cytochrome c oxidase, NADH dehydrogenase, succinate dehydrogenase, and catalase. Each of these enzymes play an important role in cellular metabolism and organism homeostasis.
Author Contributions
Academic Editor: Mezni Ali, University of Carthage, Department of Life Sciences, Carthage.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025 Andrés Concepción-Brindis, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Enzymes, as the quintessential catalysts, are integral to a multitude of biological processes, serving as catalysts for oxidation-reduction reactions. Representing the largest group of proteins, these enzymes play diverse roles in metabolism, gene expression, cell division, and critical immune system reactions. Moreover, they find applications in biotechnology industries and medical diagnostics. Enzymes account for approximately 40% of all known genes 1.
These remarkable molecules possess the ability to significantly accelerate chemical reactions, thereby facilitating coordinated metabolic pathways within cells. Furthermore, enzymes are vital for combating pathogenic organisms and mediating other biological processes 2.
Enzymes exhibit remarkable specificity in their function, with their activity and functions influenced by various factors, including their amino acid sequence, three-dimensional structure, stability, and interactions with other molecules 3. The diversity of enzyme actions and applications stems from their specificities toward substrates and reactions.
One crucial class of enzymes is oxidoreductases, which play a fundamental role in oxidation and reduction reactions. These enzymes facilitate the transfer of electrons between molecules, with the oxidized substrate serving as a hydrogen donor. Another class of enzymes, transferases, facilitate the transfer of functional groups, such as methyl or glycosyl groups, between different compounds. The catalytic capabilities of these enzymes make them key components in various biological processes 4.
In conclusion, enzymes catalyzing oxidation-reduction reactions are foundational elements in the fields of biochemistry and molecular biology. Their ability to accelerate specific chemical reactions and their functional diversity make them invaluable tools in scientific research, as well as in industrial and medical applications. The study of these enzymes and their mechanisms continues to expand our understanding of fundamental biological processes and offers opportunities for developing novel therapeutic strategies and biotechnological advancements.
Structure and catalytic mechanisms of oxidoreductases.
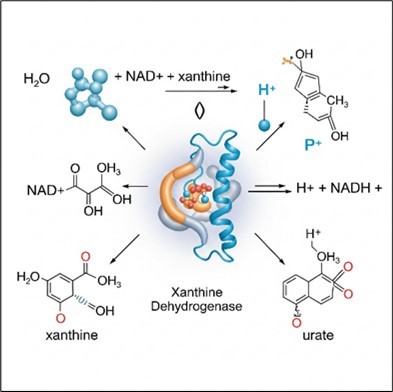
Oxidoreductases are a diverse group of enzymes that play a fundamental role in oxidation-reduction reactions within biological systems, (Figure 1).
These enzymes facilitate the transfer of electrons or hydrogen atoms, which is a process where the oxidation—the removal of electrons from a donor molecule (i.e., the reducing agent)—occurs concurrently with the reduction of an electron-acceptor molecule (i.e., the oxidizing agent), as electrons are not stable in a free state. The redox potential measures a substance's affinity for accepting electrons and becoming reduced, and electrons are transferred from substances with a lower redox potential to those with a higher redox potential. This electron transfer process is energy-yielding, and the amount of energy released is directly related to the redox potential difference between the electron donor and acceptor. Given that this energy is vital for maintaining the structure and function of living cells, oxidoreductases are crucial and highly abundant enzymes, constituting approximately one-third of all enzymatic activities registered in the BRaunschweig Enzyme Database (BRENDA) 6. One of their key functions is oxygen insertion, where they assist in incorporating molecular oxygen into organic substrates. These enzymes are classified as EC 1 within the Enzyme Commission Number classification system and, due to their vast diversity, they are further subdivided into 23 subclasses based on the specific electron donors and acceptors they utilize.
One well-known metabolic pathway that heavily relies on oxidoreductases is glycolysis. This pathway, which occurs in the cytoplasm of cells, involves a series of enzymatic reactions that ultimately convert glucose into pyruvate, generating ATP and reducing equivalents in the process. Oxidoreductases, specifically dehydrogenases, play a crucial role in glycolysis by facilitating the transfer of electrons and hydrogen atoms between different molecules 6.
Another important metabolic pathway where oxidoreductases are involved is the Krebs cycle, also known as the citric acid cycle or tricarboxylic acid cycle. This cycle takes place in the mitochondria and serves as a central hub for energy production through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. Oxidoreductases, such as the succinate dehydrogenase enzyme complex, participate in the cycle by catalyzing the transfer of electrons to the electron transport chain, which ultimately leads to ATP synthesis 7.
Speaking of the electron transport chain, this process relies heavily on oxidoreductases to transfer electrons from electron donors to electron acceptors, generating a proton gradient across the inner mitochondrial membrane. This proton gradient is then used by ATP synthase to produce ATP through oxidative phosphorylation. Oxidoreductases, such as cytochrome c oxidase, complex I, and complex II, are integral components of the electron transport chain.
In addition to their roles in energy metabolism, oxidoreductases are also involved in other important processes. For example, during photosynthesis, photosystem I and photosystem II contain oxidoreductases that facilitate electron transfer in the light-dependent reactions, leading to the production of ATP and reducing power for carbon fixation. Furthermore, oxidoreductases play a crucial role in the metabolism of fatty acids and amino acids, where they participate in the breakdown and synthesis of these molecules 8, 9, 10.
Overall, oxidoreductase enzymes are vital for the proper functioning of biological systems, participating in a wide range of oxidation-reduction reactions. Their efficiency, specificity, and biodegradability make them attractive for various industrial applications, including the production of biofuels, pharmaceuticals, and the removal of pollutants. The study of these enzymes and their mechanisms continues to be an active area of research, offering exciting possibilities for future discoveries and applications in biotechnology.
Functions and Applications of Oxidoreductases
Oxidoreductases, as key enzymes in oxidation-reduction reactions, play a fundamental role in the biochemical processes of organisms. Their ability to transfer electrons or hydrogen atoms makes them essential elements in the synthesis, degradation, and metabolism of molecules. Thanks to their biochemical properties and extensive study, these enzymes find significant applications in the food, pharmaceutical, and various chemical synthesis industries. In the near future, a promising future is envisioned for oxidoreductases as prominent biocatalysts in the industry, opening new opportunities and benefits in diverse fields 11.
In the biological context, oxidoreductases are involved in fundamental metabolic processes such as cellular respiration, photosynthesis, biosynthesis of bioactive compounds, and detoxification of metabolites. In the electron transport chain (ETC), electrons pass through a chain of proteins, increasing reduction potential and releasing energy. Complex I consists of NADH dehydrogenase, FMN, and eight Fe-S clusters. NADH from glycolysis and citric acid cycle donates electrons to FMN, then Fe-S clusters, and finally coenzyme Q, while 4 hydrogen ions pass from the matrix to the intermembrane space, contributing to the electrochemical gradient (Figure 2). Complex I may also play a role in apoptosis.
Figure 2.Schematic Diagram of Complex I.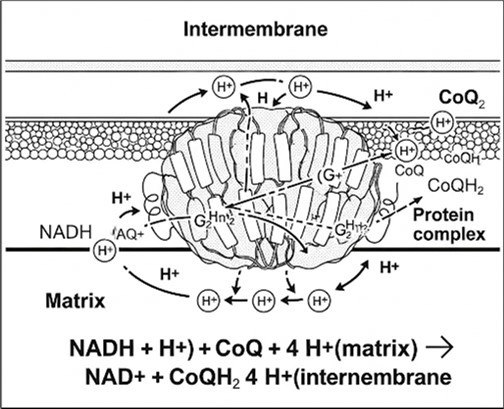
These enzymes participate in crucial steps, enabling the conversion of substrates and the transfer of electrons or hydrogen atoms. For example, in cellular respiration, oxidoreductases are key components of the electron transport chain, facilitating the flow of electrons and the generation of ATP. In photosynthesis, oxidoreductases play a vital role in capturing light energy and converting it into chemical energy through redox reactions. Moreover, oxidoreductases are involved in the biosynthesis of bioactive compounds, such as hormones and secondary metabolites, contributing to various physiological processes in organisms. Additionally, these enzymes are crucial for detoxifying harmful metabolites, ensuring the proper functioning and health of cells 12.
The analysis of oxidoreductase activities in body fluids has proven to be a valuable tool for disease diagnosis and prognosis. By measuring the activity levels of specific oxidoreductases, such as liver enzymes in blood samples, it is possible to assess liver function and detect any abnormalities or diseases affecting this organ. Similarly, changes in oxidoreductase activities in urine or other body fluids can provide insights into metabolic alterations and the status of different organs. These diagnostic applications highlight the significance of oxidoreductases as biomarkers and the importance of their accurate measurement in clinical settings 13, 14.
In the industrial field, oxidoreductases have diverse applications. These enzymes enable efficient and selective production of high-value compounds, contributing to sustainable chemical synthesis and the development of innovative pharmaceutical products. By harnessing their ability to catalyze specific redox reactions, oxidoreductases offer advantages over traditional chemical methods, such as higher selectivity, milder reaction conditions, and reduced environmental impact. This makes them valuable tools for synthesizing complex molecules, including pharmaceutical intermediates, flavors, fragrances, and specialty chemicals. Furthermore, oxidoreductases are employed in the production and modification of food products. They play a prominent role in improving organoleptic characteristics, preservation, and the production of foods with enhanced nutritional value. For instance, certain oxidoreductases are used in the brewing industry to produce specific flavors and aromas in beers, while others are employed in dairy processing to enhance the texture and flavor of cheese 9.
The potential of oxidoreductases as biocatalysts in the industry is immense, and ongoing research aims to optimize their performance and expand their applications. Advances in protein engineering and biotechnological approaches enable the modification and optimization of these enzymes for specific reactions and conditions. By tailoring their properties, such as substrate specificity, catalytic activity, and stability, oxidoreductases can be fine-tuned to meet the requirements of various industrial processes. Moreover, the exploration of novel oxidoreductases from diverse organisms, including extremophiles, presents opportunities for discovering enzymes with unique properties and applications.
In conclusion, oxidoreductases play a fundamental role in the biochemical processes of organisms, facilitating oxidation-reduction reactions and contributing to vital metabolic pathways. Their significance extends to applications in the food, pharmaceutical, and chemical synthesis industries, where they enable efficient and selective production of valuable compounds. Moreover, the analysis of oxidoreductase activities has diagnostic potential in disease assessment. The ongoing exploration and optimization of these enzymes hold promising prospects for their expanded use as biocatalysts, paving the way for innovative solutions in diverse fields.
Electron transferases
Transferases are enzymes that catalyze the transfer of functional groups through nucleophilic substitution reactions. Their industrial application shows promise, particularly in the use of glycosyltransferases for synthesizing oligosaccharides involved in cellular recognition. Another relevant example is the combination of glucokinase, a transferase, with acetate kinase to produce glucose-6-phosphate (Figure 3).
Figure 3.Transfer of molecules from donor to recipient.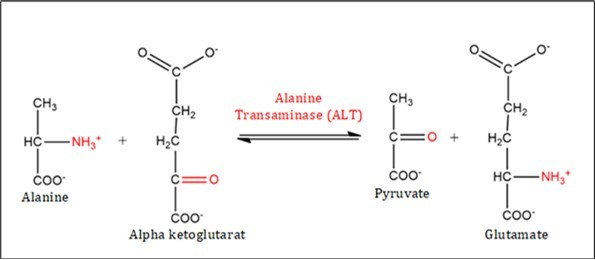
These examples illustrate the versatility and utility of transferases in generating bioactive compounds. As our understanding of their mechanisms deepens, it is expected that their industrial application will expand, offering opportunities for synthesizing valuable compounds and molecules across diverse industries 15.
Glycosyltransferases (GTs) are a prominent subclass of transferases that play a crucial role in the formation of glucosidic bonds in living organisms. These enzymes exhibit high specificity in the synthesis, degradation, and modification of carbohydrates, contributing to the vast chemical diversity found in oligosaccharides and glycoconjugates 16.
GTs act by transferring sugar moieties from activated donor molecules to specific acceptor molecules, leading to the formation of glycosides in monosaccharides, oligosaccharides, or polysaccharides. The donor substrates utilized by GTs include nucleotide sugars, sugar phosphates, lipids, and sugar phosphates, while the acceptor substrates encompass carbohydrates, proteins, lipids, DNA, and other small molecules 17.
Beyond their fundamental biological function, GTs are involved in various cellular processes such as cellular interactions, development, diseases, and immune defense. The formation of the glucosidic bond can occur with retention or inversion of the anomeric carbon configuration of the donor substrate. The precise mechanism of retention is still a subject of debate, although an "orthogonal associative" mechanism has been proposed based on crystallographic evidence. The study of GTs is of utmost importance for understanding carbohydrate synthesis, unraveling the intricacies of cellular interactions, and developing novel therapeutic approaches 18, 19.
The industrial application of GTs holds immense potential for the synthesis of valuable compounds. By harnessing the specificity and catalytic capabilities of these enzymes, it becomes possible to produce complex carbohydrates with precise structures and functional properties. This has significant implications in various industries, including pharmaceuticals, where specific glycoconjugates can serve as therapeutic agents or vaccine components. Additionally, the production of functional oligosaccharides through enzymatic synthesis offers new avenues for the development of prebiotics, food additives, and ingredients with health benefits 15.
Furthermore, the optimization of GTs through protein engineering approaches allows for the modification of their substrate specificity, catalytic efficiency, and stability. This enables the generation of tailor-made transferases with enhanced properties for specific applications. The integration of GTs with other biocatalysts and chemical processes opens up opportunities for multi-step enzymatic cascades, leading to more efficient and sustainable production routes for complex molecules 19.
Particularly transferases of type glycosyltransferases, are versatile enzymes with significant industrial potential. Their ability to catalyze the transfer of functional groups enables the synthesis of complex carbohydrates and bioactive compounds with high specificity. The ongoing research on GTs and other transferases expands our understanding of their mechanisms and opens opportunities for their application in diverse industries. By harnessing their catalytic power, it becomes possible to develop novel therapies, create valuable compounds, and improve processes in pharmaceuticals, biotechnology, and food production. The future of transferase-based biocatalysis holds great promise in advancing sustainable and efficient chemical synthesis while providing new solutions to address societal challenges.
Enzymatic electrosynthesis in biological–inorganic hybrid systems offers a sustainable route for chemical production, exploiting renewable energy and highly selective catalysts active under ambient conditions. Despite its promise, progress is hindered by limited insight into the molecular basis of electrode-mediated electron transfer and biocatalysis. Insights from non-electrosynthetic electron transfer proteins reveal precise control of redox potential and targeted delivery of electrons via spatially separated redox sites connected by cofactor arrays functioning as insulated electron relays. Electron migration occurs through quantum tunneling over nanometer-scale distances, exemplified by the Shewanella oneidensis Mtr conduit, which transfers electrons across the cell wall to extracellular acceptors such as solid ferrihydrite. Understanding these biochemical strategies is essential for advancing the structural biology of enzymatic electrosynthesis and optimizing its technological applications (Figure 4) 19.
Figure 4.Electron transfer mechanism in redox proteins.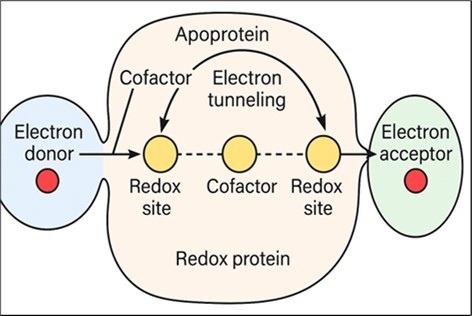
Regulation of redox enzymes
Redox signaling acts as a critical mediator in the dynamic interactions between organisms and their external environment, profoundly influencing both the onset and progression of various diseases. Under physiological conditions, oxidative free radicals generated by the mitochondrial oxidative respiratory chain, endoplasmic reticulum, and NADPH oxidases can be effectively neutralized by NRF2-mediated antioxidant responses. These responses elevate the synthesis of superoxide dismutase (SOD), catalase, as well as key molecules like nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione (GSH), thereby maintaining cellular redox homeostasis 20.
The regulation of redox enzymes is crucial for maintaining the delicate redox balance in biological systems. These enzymes play a pivotal role in electron transfer and the regulation of metabolic pathways. Their activity is modulated through various mechanisms, including phosphorylation, binding of cofactors, and interaction with regulatory proteins 21, 22.
Biological redox reactions exhibit a dual nature, capable of promoting both physiological signaling responses and pathophysiological signals. In this context, endogenous antioxidant molecules play a crucial role. One notable example is glutathione, an essential cellular nucleophile that interacts with oxidants and forms networks with other critical enzymes such as peroxiredoxins. The Nrf2-Keap1 pathway is an exemplary transcriptional antioxidant response, wherein this type of response plays a significant role in endoplasmic reticulum stress and ischemia-reperfusion processes. Understanding these mechanisms provides a foundation for developing antioxidant therapeutic interventions in redox-related diseases 23, 24.
However, there is still much to learn about the intricacies of these signaling processes, the antioxidant responses involved, and the thresholds at which redox perturbations are disrupted. Additionally, the mechanisms underlying the transport or interconversion of reactive oxygen species (ROS) inside or outside cellular compartments, and how these conversions contribute to cellular homeostasis, remain largely unknown. It is anticipated that the coming years will bring forth additional examples or uncover new and alternate mechanisms that contribute to redox balance, antioxidant defense, and ROS/peroxide signaling (Table 1) 25, 26.
Table 1. Reactive oxygen species| Nomenclature | Structure |
|---|---|
| Hydroxyl radical | (HO•) |
| Hydroxide ion | (HO−) |
| Triplet oxygen | (O22•) |
| Superoxide anion | (O2•−) |
| Peroxide ion | (O22-) |
| Hydrogen peroxide | (H2O2) |
| Nitric oxide | (NO•) |
Further research is necessary to unravel the complex interplay between redox enzymes, antioxidant molecules, and signaling pathways. Advances in analytical techniques and omics technologies have provided valuable tools for investigating redox dynamics at the molecular level. For instance, the development of redox-sensitive probes and sensors has enabled real-time monitoring of redox changes in specific cellular compartments. High-throughput techniques, such as proteomics and metabolomics, offer a comprehensive view of redox-related changes occurring in response to physiological or pathological stimuli.
Moreover, studying redox enzymes and their regulation opens up opportunities for therapeutic interventions. By targeting specific redox enzymes or modulating their activity, it becomes possible to restore redox balance in disease conditions characterized by oxidative stress. Small molecules, such as enzyme activators or inhibitors, can be designed to selectively modulate the activity of key redox enzymes, thereby influencing cellular redox status and associated signaling pathways. Such interventions hold promise for the development of novel treatments for a wide range of diseases, including neurodegenerative disorders, cardiovascular diseases, and cancer 27.
In conclusion, the regulation of redox enzymes is crucial for maintaining redox balance and cellular homeostasis. Understanding the intricate mechanisms that govern redox signaling and antioxidant responses is essential for developing therapeutic strategies to combat redox-related diseases. Advancements in analytical techniques and our expanding knowledge of redox biology provide exciting opportunities to uncover new insights and develop targeted interventions. Continued research in this field holds great promise for improving human health and addressing the challenges posed by oxidative stress and redox dysregulation.
Advancements and future prospects in the study of redox enzymes
Advancements that are crucial for the development of highly efficient cell factories in sustainable bioproduction have been explored, with a focus on harnessing these advancements to enhance the study and application of redox enzymes in various scientific and technological fields 28, 29.
One area of advancement lies in the engineering of redox cofactor-dependent enzymes using auxotrophic strains of redox cofactors. By manipulating the availability of specific cofactors, it becomes possible to fine-tune the activity and properties of enzymes. This approach has shown promise, as significant improvements can often be achieved with just one round of mutagenesis. Optimizing key properties such as substrate specificity, catalytic rate, and stability, including thermostability, is essential for their commercial applications. Moreover, the development of non-canonical auxotrophic strains of redox cofactors holds great potential for accelerating the engineering of efficient and robust biocatalysts 30, 31, 32.
In the pursuit of these advancements, a wide variety of protein engineering approaches have been developed. These methodologies aim to identify improved enzyme variants through screening and/or selection processes. Directed evolution, for instance, allows for the generation of diverse enzyme libraries, which can be subjected to screening or selection to identify variants with desired properties. Rational design approaches, on the other hand, utilize structural information and computational tools to guide the modification of enzyme active sites or other regions critical for function. By combining these approaches and utilizing high-throughput screening technologies, researchers can rapidly explore the vast sequence space and identify enzyme variants with enhanced catalytic properties or novel functionalities 33, 34.
The application of these protein engineering approaches extends beyond improving the performance of individual enzymes. They also enable the design of enzyme cascades and metabolic pathways for the efficient conversion of substrates into valuable products. By strategically selecting and engineering redox enzymes, it becomes possible to optimize the entire biocatalytic system, enhancing its efficiency, productivity, and sustainability. Additionally, advancements in synthetic biology, metabolic engineering, and systems biology contribute to the development of efficient cell factories capable of producing high titers, yields, and productivities of desired compounds 29.
These advancements in redox enzyme engineering and bioproduction have far-reaching implications. They hold promise for the development of sustainable and economically viable biotechnological processes, including the production of biofuels, pharmaceuticals, specialty chemicals, and renewable materials. Redox enzymes, with their ability to facilitate oxidation-reduction reactions and control redox balance, are key players in these processes. Furthermore, the integration of redox enzymes into cell factories and biorefineries offers opportunities for the utilization of renewable feedstocks, minimizing the reliance on fossil resources and reducing environmental impact 34.
The study and application of redox enzymes have undergone significant advancements, enabling the development of highly efficient cell factories and bioproduction processes. Engineering redox cofactor-dependent enzymes, utilizing auxotrophic strains, and employing protein engineering approaches are among the strategies employed to optimize enzyme properties and improve overall biocatalytic systems. These advancements have the potential to revolutionize various scientific and technological fields, paving the way for sustainable bioproduction and the development of novel biotechnological solutions. Continued research and exploration in this area are essential for unlocking the full potential of redox enzymes and realizing their impact on a global scale.
Engineering of redox enzymes to improve their activity and stability
The advancements made so far are of utmost importance; however, there are still unexplored areas of research in the growth-coupling based on redox cofactors (Figure 5).
Figure 5.Electron transport by redox cofactors.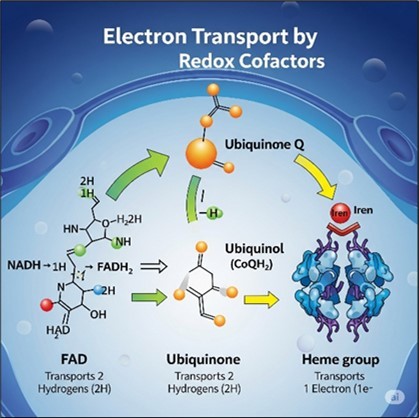
The engineering of cofactor auxotrophic strains in organisms other than Escherichia coli, such as Corynebacterium glutamicum and Pseudomonas putida, is proposed, representing the first cofactor auxotrophic strains in these species. These strains could be utilized for enzyme engineering, opening the possibility of growth-coupled enzyme engineering in different hosts. Moreover, other industrially relevant hosts such as Ralstoniaeutropha, Clostridium sp., Yarrowialipolytica, and Saccharomyces cerevisiae could be suitable candidates for similar engineering platforms. The construction of orthogonal strains from different species could be easily achieved 35, 36.
The coupling of growth through oxidation or reduction rates of cofactors presents an opportunity for engineering the biocatalysis of products that do not meet the requirements of other high-performance enzyme engineering approaches. These systems can overcome obstacles in engineering metabolic pathways or enzymes involved in the sustainable production of gases, aliphatic hydrocarbons, and organic solvents. Additionally, they allow for the modification of cofactor specificity, the discovery of novel catalytic capabilities, and the optimization of the expression of heterologous enzymes 36, 37, 38.
These growth-coupling platforms are not limited to specific enzyme engineering. Any enzyme or metabolic pathway utilizing cofactors can be targeted for engineering. In addition to enzyme engineering, growth-dependent strains can serve as useful hosts for bioproduction, eliminating the need for antibiotic selection and addressing biological safety concerns 39, 40, 41.
The utilization of non-canonical redox cofactors remains a relatively unexplored area in metabolic engineering. Its development would enable precise control of electron flux and high-yield biological production. Engineering relevant enzymes to utilize non-canonical redox cofactors can be expedited by employing auxotrophic strains for these cofactors and utilizing growth-coupling approaches 42, 43.
The growth-coupling based on redox cofactors offers opportunities for enzyme and metabolic pathway engineering, particularly in the sustainable production of chemicals. Engineering cofactor auxotrophic strains in different hosts and exploring non-canonical redox cofactors are promising areas of research in metabolic engineering.
On the other hand, there are other types of enzymes, alternative oxidase (AOX) regulates the level of reactive oxygen species and nitric oxide (NO) in plants. While it alleviates NO formation under normoxic conditions, there are several indications that in conditions of low oxygen in plants AOX can be involved in the production of NO from nitrite. This results in the facilitation of glycolytic reactions by reoxidation of the glycolytically formed NADH and diverting the glycolytic carbon toward the formation of alanine and other amino acids. Pyruvate formed in glycolysis can activate AOX and facilitate its operation under these conditions. It is concluded that AOX is an important player in the hypoxic response in plants that regulates the redox level by participating in NO turnover as a nitrite: NO reductase in cooperation with nitrate reductase and phytoglobin 44.
Conclusions
In summary, enzymes play a fundamental role in numerous biological processes by catalyzing oxidation-reduction reactions. Representing the largest group of proteins, these highly specific molecules exhibit remarkable ability to significantly accelerate chemical reactions, thus serving as valuable tools in scientific research, industry, and medicine. Oxidoreductases, a class of enzymes, are key players in oxidation-reduction reactions in biological systems, playing a crucial role in the synthesis, degradation, and metabolism of molecules, as well as in metabolic processes. These enzymes utilize cofactors such as NAD, FAD, or NADP and are essential in cellular respiration, photosynthesis, biosynthesis of bioactive compounds, and detoxification of metabolites.
Additionally, other classes of oxidoreductase enzymes, including peroxidases, hydroxylases, oxygen ases, and reductases, have significant roles in both aerobic and anaerobic metabolism. Transferases, another class of enzymes, catalyze the transfer of functional groups, such as glycosyl groups, and hold promising applications in synthesizing bioactive compounds and generating chemical diversity in oligosaccharides and glycoconjugates. The regulation of redox enzymes is crucial for maintaining redox balance in biological systems, and the study of antioxidant responses and redox signaling remains an ongoing area of research.
Advances in the engineering of redox enzymes, including the utilization of cofactor auxotrophic strains and growth coupling, have opened new possibilities for sustainable chemical production. The exploration of non-canonical redox cofactors and the development of orthogonal strains could expedite the engineering of efficient and robust biocatalysts. Overall, the study and application of redox enzymes offer exciting prospects for metabolic engineering and the advancement of sustainable industrial production. Further research is needed in these areas to gain a deeper understanding of the mechanisms involved and fully harness their potential in the generation of valuable chemicals and biomolecules.
References
- 1.McLure R J, Radford S E, Brockwell D J. (2022) High-throughput directed evolution: a golden era for protein science. Trends Chem. 4, 378-391.
- 2.Schomburg L J, Ulbrich M, Placzek S, Chang A, Schomburg D. (2017) The BRENDA enzyme information system - from a database to an expert system. DOI: https://doi.org/10.1016/j.jbiotec.2017.04.020 , J. Biotechnol 261, 194-206.
- 3.CER Reis, N, HBS Bento, AKF Carvalho, Vandenberghe L P et al. (2023) Aminabhavi TM, Chandel AK. Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries. , Chem. Eng. J 451-138690.
- 4.Worthington C C, Worthington V, Worthington a. Worthington Biochemical Corporation (2019) Introduction to Enzymes.
- 5.Chapter. (2020) Biological Application and Disease of Oxidoreductase Enzymes. DOI: http://dx.doi.org/10.5772/intechopen.93328
- 6.Prešern U, Goličnik M. (2023) Enzyme Databases in the Era of Omics and Artificial Intelligence. , International Journal of Molecular Sciences 24, 16918-10.
- 7.McDonald A. (2019) The Enzyme List Class 1-Oxidoreductases. Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) .
- 8.Wu S, Snajdrova R, Moore J C, Baldenius K, Bornscheuer U T. (2021) Biocatalysis: enzymatic synthesis for industrial applications. , Angew. Chem. Int. Ed 60, 88-119.
- 9.D A Cantrell. (2022) Protein synthesis, degradation, and energy metabolism in T cell immunity. , Cell. Mol. Immunol 19, 303-315.
- 10.Ramsay R R. (2019) Electron carriers and energy conservation in mitochondrial respiration. , ChemTexts 5, 9.
- 11.Martínez A T, Ruiz-Dueñas F J, Camarero S, Serrano A, Linde D et al. (2017) Oxidoreductases on their way to industrial biotransformations. Biotechnology Advances. 35, 815-831.
- 12.Chapman J, Ismail A E, Dinu C Z. (2018) Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts. 8, 238.
- 13.Manjrekar S, Wadekar T, Sumant O. (2020) Enzymes Market Type (Protease, Carbohydrase, Lipase, Polymerase and Nuclease, and Other Types), Source (Microorganisms, Plants, and Animals), Reaction Type (Hydrolase, Oxidoreductase, Transferase, Lyase, and Other Reaction Types), and Application (Food and Beverages, Household Care, Bioenergy, Pharmaceutical and Biotechnology, Feed, and Other Applications)—Global Opportunity Analysis and Industry Forecast.
- 14.Giannina E, Atalah J, Blamey J M. (2021) Extremophilic Oxidoreductases for the Industry: Five Successful Examples With Promising Projections. Frontiers in Bioengineering and Biotechnology. 9, 710035-10.
- 15.PEV Paul, Sangeetha V, Deepika R G. (2019) . Emerging Trends in the Industrial Production of Chemical Products by Microorganisms. 2. Microbial biotechnology 107-125.
- 17.Zhao M, Zhu Y, Wang H, Xu W, Zhang W et al. (2023) An overview of sugar nucleotide-dependent glycosyltransferases for human milk oligosaccharide synthesis. , J. Agric. Food Chem 33, 12390-12402.
- 18.BAH Smith, Bertozzi C R. (2021) The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. , Nat. Rev. Drug Discov 3, 217-243.
- 19.Lienemann M. (2021) Molecular mechanisms of electron transfer employed by native proteins and biological-inorganic hybrid systems. , Computational and Structural Biotechnology Journal 19, 206-213.
- 20.Li B, Ming H, Qin S, E C Nice, Dong J et al. (2025) Redox regulation: mechanisms, biology and therapeutic targets in diseases. Signal Transduction and Targeted Therapy. 10(1), 10-1038.
- 21.Fridovich I. (1995) Superoxide radical and superoxide dismutase. Annual Review of Biochemistry. DOI: https://doi.org/10.1146/annurev.bi.64.070195.000525 64, 97-112.
- 22.Das P, Sen P. (2024) Relevance of Oxidoreductases. in Cellular Metabolism and Defence. IntechOpen 10-5772.
- 23.Lu X, He Z, Xiao X, Wei X, Song X et al. (2023) Natural Antioxidant-Based Nanodrug for Atherosclerosis Treatment. , Small 49-2303459.
- 24.Wang L, Liu C, Lu W, Xu L, Kuang L et al. (2023) ROS-sensitive Crocin-loaded chitosan microspheres for lung targeting and attenuation of radiation-induced lung injury. Carbohydr Polym. 307: 120628. doi: 10.1016/j.carbpol.2023.120628. Epub 36781279.
- 25.Jomova K, Alomar S Y, Alwasel S H, Nepovimova E, Kuca K et al. (2024) Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. , Arch Toxicol 5, 1323-1367.
- 26.Sachdev S, Ansari S A, Ansari M I, Fujita M, Hasanuzzaman M. (2021) Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. , Antioxidants 10, 277.
- 27.Drăgoi C M, Diaconu C C, Nicolae A C, Dumitrescu I B. (2024) Redox Homeostasis and Molecular Biomarkers in Precision Therapy for Cardiovascular Diseases. Antioxidants. 10, 1163-10.
- 28.Bouzon M, Döring V, Dubois I, Berger A, GMM Stoffel et al. (2021) Change in cofactor specificity of oxidoreductases by adaptive evolution of an escherichia coli nadph-auxotrophic strain. MBio. 12-4.
- 29.Chen J, Wang Y, Zheng P, Sun J. (2022) Engineering synthetic auxotrophs for growth-coupled directed protein evolution. Trends Biotechnol. DOI: https://doi.org/10.1016/J.TIBTECH.2022.01.010
- 30.Black W B, Li H. (2022) Cell-free noncanonical redox cofactor systems. , Methods Mol. Biol 2433, 185-198.
- 31.King E, Maxel S, Li H. (2020) Engineering natural and noncanonical nicotinamide cofactor-dependent enzymes: design principles and technology development. DOI: https://doi.org/10.1016/J.COPBIO.2020.08.005 , Curr. Opin. Biotechnol 66, 217-226.
- 32.Liu Y, Feng Y, Wang L, Guo X, Liu W et al. (2019) Structural insights into Phosphite dehydrogenase variants favoring a non-natural redox cofactor. ACS Catal. 9(3), 1883-1887.
- 33.Scown C D. (2022) Keasling JD. Sustainable manufacturing with synthetic biology. Nature Biotechnology. 40(3), 304-307.
- 34.Weusthuis R A, Folch P L, Pozo-Rodríguez A, Paul C E. (2020) Applying noncanonical redox cofactors in fermentation processes. In: iScience. , Elsevier Inc 23, 10-1016.
- 35.Sekar B S, Seol E, Park S. (2017) Co-production of hydrogen and ethanol from glucose in Escherichia coli by activation of pentose-phosphate pathway through deletion of phosphoglucose isomerase (pgi) and overexpression of glucose-6-phosphate dehydrogenase (zwf) and 6-phosphogluconate dehyd. Biotechnology for Biofuels. 10-1.
- 36.Wenk S, Schann K, He H, Rainaldi V, Kim S et al. (2020) An “energy-auxotroph” Escherichia coli provides an in vivo platform for assessing NADH regeneration systems. , Biotechnol. Bioeng 117, 3422-3434.
- 37.King E, Maxel S, Li H. (2020) Engineering natural and noncanonical nicotinamide cofactor-dependent enzymes: design principles and technology development. DOI: https://doi.org/10.1016/J.COPBIO.2020.08.005 , Curr. Opin. Biotechnol 66, 217-226.
- 38.Black W B, Zhang L, Mak W S, Maxel S, Cui Y et al. (2019) Engineering a nicotinamide mononucleotide redox cofactor system for biocatalysis. Nature Chemical Biology. 16(1), 8794-10.
- 39.Chen J, Wang Y, Zheng P, Sun J. (2022) Engineering synthetic auxotrophs for growth-coupled directed protein evolution. Trends. in Biotechnology.773-776
- 40.Black W B, Li H. (2022) Cell-free noncanonical redox cofactor systems. , Methods Mol. Biol 185-198.
- 41.Liu J, Li H, Zhao G, Caiyin Q, Qiao J. (2018) Redox cofactor engineering in industrial microorganisms: strategies, recent applications and future directions. , J. Ind. Microbiol. Biotechnol 45(5), 313-327.
- 42.Zhang L, King E, Black W B, Heckmann C M, Wolder A et al. (2022) Directed evolution of phosphite dehydrogenase to cycle noncanonical redox cofactors via universal growth selection platform. Nature Communications. 13, 1-12.