Abstract
The paper explores the formation of a-oxoaldehydes during the interaction of glucose metabolites with hydroxyl or alkoxyl radicals. Hydroxyl radicals were generated under radiolysis of aqueous solutions, and alkoxyl radicals (t-BuO) were obtained in the model system tert-butyl hydroperoxide/Fe2+. High-performance liquid chromatography revealed that methylglyoxal was one of the organic products resulting from t-BuO-induced transformations of fructose-1,6-bisphosphate under hypoxic conditions. The interaction of lysine and methylglyoxal one of the main targets of a-oxoaldehydes in proteins was also studied. As chemiluminescence and EPR spectroscopy demonstrated, this reaction generates a methylglyoxal anion radical, a cation-radical of methylglyoxal dialkylamine and a superoxide anion radical. EPR signal of methylglyoxal-derived free radicals was observed in hypoxia, whereas only the trace amounts of these free radicals were recorded in the aerated reaction medium.
Author Contributions
Academic Editor: Jie Yin, Institute of Subtropical Agriculture and University of Chinese Academy of Sciences, China.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 V.Z. Lankin, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Ionizing irradiation or metabolic disturbances in the living body can lead to hyperproduction of reactive oxygen species (ROS), which promotes the activation of free radical processes – the phenomenon which can damage some biologically significant molecules 1, 2, 3. Oxidative stress is also an important factor in the pathogenesis of atherosclerosis and diabetes mellitus 4, 5, 6, 7. The elevation of the generated ROS in atherosclerosis against the background of hyperlipidemia leads to a rapid accumulation of lipohydroperoxides in blood as well as in the vascular wall 6, 8. In the process of free radical peroxidation of the unsaturated lipids lipohydroperoxides (primary oxidation products) undergo oxidative degradation with the formation of reactive carbonyl compounds − aldehydessuch as 4-hydroxynonenal and malondialdehyde (MDA) 4, 9, 10.
Substances with phosphoether bonds in their structure play an important role in biosystems. Such compounds as fructose-1,6-bisphosphate (Fru-1,6-P2) and glyceraldehyde-3-phosphate (G-3-P) are glucose (Glc) metabolites which are formed during glycolysis. Since the interaction of oxygen-centered radicals with the monosaccharides leads to the formation of physiologically reactive carbonyl and dicarbonyl compounds 11, monosaccharide phosphates and their analogues. The latter ones being the main metabolites of glycolysis can be assumed to undergo the similar free radical transformations, which form α-oxoaldehydes.
Thus, oxidative stress, accompanied by an increased level of organic hydroperoxides, inevitably leads to a carbonyl stress, characterized by the accumulation of reactive carbonyl species. In diabetic hyperglycemia Glc gets autooxidated, which is accompanied by the formation of MDA homolog - glyoxal 10. This process may be increased by a co-oxidation of unsaturated lipids and Glc 12, 13, 14. The structural isomer of MDA, methylglyoxal is formed in diabetes as a result of enzymatic oxidation of triose phosphate accumulated during glycolysis 4, 10, 15.
The potential danger of accumulating dicarbonyls during carbonyl stress development in diabetes lies in the ability of aldehyde groups to react with amino groups of proteins in order to form inter- and intramolecular Schiff bases cross-links , since this process triggers the biopolymer modification and the consequent violation of their normal functioning 4, 9, 10, 15. In particular, modifying the terminal ε-residues of L-lysine in the molecule of apoprotein B-100 of low-density lipoproteins (LDL) by dicarbonyls increases the capture of LDL particles by vascular wall cells with the help of scavenger receptors and leads to a pro-atherogenic vascular damage 16. In addition, literature on the subject mentions about the formation of free radical intermediates during the interaction of methylglyoxal with L-alanine and albumin 17, 18; noteworthily, the mechanism of this reaction has not been fully ascertained so far.
In the present study we investigate the possibility that the metabolites of glycolysis can create the non-enzymatic formations of methylglyoxal, as well as the possibility of ROS and other free radical products to form in the process of interaction between this high reactive dicarbonyl compound and amino acids.
Materials and Methods
The following reagents produced by Sigma-Aldrich (USA) were used in the present work: superoxide dismutase from bovine erythrocytes, fructose (Fru), fructose-1,6-bisphosphate (Fru-1,6-P2), Glc, glucose-1-phosphate (G-1-P), glucose-6-phosphate (G-6-P), G-3-P, glyceraldehyde, methylglyoxal and glyoxal (40% water solutions), formaldehyde (37% water solution), potassium dihydrogen phosphate, tert-butylhydroperoxide (t-BuOOH), L-lysine, lucigenin, o-phenylenediamine (OPDA), 2,4-dinitrophenylhydrazine (DNPH), nitro blue tetrazolium (NBT), 1,1,3,3-tetraethoxypropane.
Twice-distilled water was used to prepare aqueous solutions for the compounds under investigation. Aqueous 0.1 M and 0.01 M solutions of the glucose metabolites have been saturated with argon (Ar) or oxygen (O2) of a high purity for 30−45 minutes. The prepared samples were irradiated in a g-unit with a 60Co source. The absorbed dose range, equaled to 0.13÷1.92 kGy, was determined by Fricke dosimetry using a radiation chemical yield G(Fe3+) = 16.2´10-7mol/J 19. In all other experiments the mixture consisted of t-BuOOH/Fe2+ with a different molar concentration of t-BuOOH was added to the substrate solutions. In the latter case the conversion products of the substrates and t-BuOOH was determined 15 minutes after the solutions had been mixed.
Dicarbonyls (a-oxoaldehydes) were analysed employing the method of a high-performance liquid chromatography using LCMS-2020 chromatograph ("Shimadzu", Japan) after the preliminary derivatization of the samples with OPDA. Test samples were mixed with OPDA solution and have been held for 12 hours in a dark place to obtain the corresponding quinoxalins (Figure 1A). To effectively separate the quinoxalines formed from methylglyoxal and glyoxal, their gradient elution was conducted using methanol and water. The separation was carried out on a Shim-pack VP-ODS column. To identify a-oxoaldehydes, UV-detector (λmax = 315 nm) and mass spectrometric detector (M+H+ at m/z 131 for glyoxal; at m/z 145 for methylglyoxal) were used.
Carbonyls were analysed by a high-performance liquid chromatography using LCMS-2020 chromatograph ("Shimadzu", Japan) after the preliminary derivatization of the samples with DNPH (Figure 1B). Chromatographic conditions: Shim-pack VP-ODS column; a mobile phase of methanol/water; UV-detector (λmax = 366 nm).
Figure 1.Derivatization of dicarbonyls and carbonyls using o-phenylenediamine and 2,4-dinitrophenylhydrazine, respectively
To determine the inorganic phosphate, solutions of (NH4)2MoO4 in 1 M H2SO4 and 10% solution of FeSO4•7H2O in 7.5 mM H2SO4 (phosphate concentration more than 1•10-5mol/L) or solutions of (NH4)2MoO4, (SbO)KC4H4O6•0.5H2O in 9M H2SO4 and ascorbic acid (phosphate concentration less than 1•10-5mol/L) were used. The concentration of inorganic phosphate ions was determined using a reagent-spectrophotometric method based on the formation of a yellow heteropoly acid during the reaction of orthophosphates with ammonium molybdate in an acid medium, which turns into an intensely stained blue compound (with the addition of Fe2+ λmax=720 nm, with the addition of Sb2+ λmax=880 nm) under the influence of reducing agents , on the SPECORD S600 spectrophotometer ("Analytik Jena", Germany).
Hydroperoxide-induced yields (Y, molecule/100 initiator molecules) were determined from the ratio between the concentration of the conversion products of Fru-1,6-P2 and the sum of the concentrations of acetone and tert-butanol formed from t-BuOOH.
Radiation-chemical yields (G, 107 mol/J) of the formation of reaction products were determined in the linear sections of the dependence of the concentrations of substances on the absorbed dose. The results obtained in three independent experiments were used to calculate G and Y values. The error in radiation- and hydroperoxide-induced yields were estimated using the least squares method with the confidence coefficient value of 0.95.
EPR spectra were recorded at a room temperature in an E-109E spectrometer (“Varian”, USA). Recording settings were as follows: microware power 20 mW, microware frequency 9.15 GHz, high frequency modulation amplitude 0.2 mT. Recording of the spectra was started 1 min after the reaction components had been mixed. The reaction mixture (80 μl) was introduced into PTFE Sub-Lite-Wall capillary tubing (inside diameter 0.635 mm, wall thickness 0.051 mm) (“Zeus Industrial Products”, USA). The capillaries were folded four times and inserted into an EPR quartz tube, open at both ends, which allowed for an adequate gas flow (a mixture of O2 and N2) around the sample in EPR TE102 cavity. EPR signal of the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was used as standard 20, 21. EPR spectra were simulated by SimFonia software (“Bruker”, Germany).
Generation of superoxide anion radical (О2·-) was detected using two independent methods: reduction of NBT by superoxide and О2·- induced chemiluminescence of lucigenin. The kinetics of the accumulation of NBT reduction product, formazan, was determined by absorption at 560 nm in a Hitachi-557 spectrophotometer (Japan) at 25°C. To initiate the reaction methylglyoxal was added to the NBT-containing medium while L-lysine – to carbonate buffer (0,1М, pH 9.5). Chemiluminescence was measured by a Lum-5773 chemiluminometer (Russia) in the medium containing lucigenin (20 μM), L-lysine (0.015 M), and methylglyoxal (0.015M) in K,Na-phosphate buffer (0.1 M, pH 7.8). Measurements were performed at 37°C under the continuous stirring of the reaction medium. Statistical treatment of the data was performed using Student’s t-criterion.
Results and Discussion
The Formation of Methylglyoxal and Other Products During the Degradation of Dlucose Metabolites Induced by Various Free Radicals
One of the main reasons behind the study of free radical processes is an extremely important and contradictory role they play in living systems. Lipid peroxidation is the most well-studied process of damaging biologically important substances, since the resultant product of this process – polyunsaturated fatty acid residues – are transformed with the formation of toxic oxidation products and oxidative degradation 1, 8, 9, 10.
In our work we used a steady-state radiolysis technique to generate free radicals . It is free from the artifacts characteristic of the reagent-based methods. When exposed to radiation, water undergoes a breakdown sequence into hydroxyl radicals and other radical and molecular products (Figure 2).
Figure 2.The main products of water radiolysis.
Hydroxyl radicals (·OH) are highly reactive and consequently short-lived (in vivo their half-life is approximately 10−9 seconds 23). They can also occasionally appear as byproducts of the immune response in living organisms (by macrophages and microglia) or in the mitochondria 3, 24. Hydroxyl radicals can damage virtually all types of macromolecules: carbohydrates, nucleic acids, lipids and amino acids, which makes them very dangerous for the organism. Unlike superoxide, which can be detoxified by superoxide dismutase, hydroxyl radicals cannot be eliminated by an enzymatic reaction.
Our previous studies 25, 26, 27 proved that the fragmentation of hydroxyl-containing organic compounds procceding in parallel with (proceeding vis-à-vis) the formation of carbon-centered radicals is possible under hypoxic conditions (Figure 3).
Figure 3.The mechanism of carbon-centered radicals formed in the reaction of hydroxyl radicals with organic compounds containing OH-group fragmentation 252627.
Oxygen- and nitrogen-centered radicals formation can also trigger the destruction of the biomolecules’ carbon skeleton 11, 27, 28.
The data presented in Table 1 show the radiation-chemical yields of the major radiolysis products formed from Glc, its metabolites (Fru-1,6-P2, G-3-P and Glc-6-P), as well as the reference compounds (Fru, Glu, Glc-1-P) in aqueous solutions.
Table 1. Radiation-chemical yields (G, 107 mol/J) of the major radiolysis products formed in the sugars under the investigation and the aqueous solutions, their derivatives| System | G (products), 107mol/J | ||||
| Compound | Deaerated /oxygenated | Inorganicphosphate | Methylglyoxal | Glyoxal | Formaldehyde |
| G-3-P (0.003 М) | Ar | 2.21±0.17 | 0.11±0.03 | 0.02±0.01 | - |
| Fru-1,6-P2 (0.01 M) | Ar | 3.41±0.21 | 0.02±0.01 | 0.03±0.01 | 0.06±0.02 |
| Fru-1,6-P2 (0.1 M) | Ar | 6.81±0.17 | 0.04±0.01 | 0.11±0.02 | 0.02±0.01 |
| O2 | 2.99±0.70 | n/d* | n/d | - | |
| Fru (0.1 M) | Ar | - | 0.09±0.02 | 0.02±0.01 | 0.14±0.04 |
| O2 | - | n/d | 0 | 0.40±0.11 | |
| Glc (0.1 M) | Ar | - | n/d | 0.04±0.01 | - |
| Glc-1-P (0.1 M) | Ar | 2.88±0.12 | n/d | 0.03±0.01 | - |
| Glc-6-P (0.1 M) | Ar | 1.34±0.14 | n/d | 0.06±0.02 | - |
As is well-known, the excessive formation of lipohydroperoxides, which in the Fenton-type reaction forms alkoxyl radicals (LO·), is associated with the concept of ferroptosis 22. In the present study we used t-BuOOH/Fe2+ model system for alkoxyl radicals generation (Figure 4).
Figure 4.Generation of alkoxyl radicals (A) and the products of their further transformations (acetone and tert-butanol) (B) in a model system containing t-BuOOH and Fe2+ ions.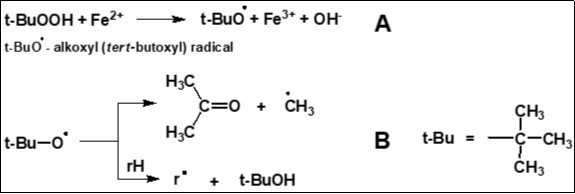
The data presented in Figure 5A show the hydroperoxide-induced yields (Y, molecule/100 initiator molecules) of the major products formed in Fru-1,6-P2 aqueous 0.01 M deaerated solutions in the presence of t-BuOOH/Fe2+.
As we can see from the data presented in Table 1, the main product of free radical transformations of Fru-1,6-P2, G-3-P, Glc-1-P and Glc-6-P initiated by ·OH is an inorganic phosphate. The same can be stated for t-BuO·-induced transformations of Fru-1,6-P2 (Figure 5A). Among other products of the transformations induced by hydroxyl and tert-butoxyl radicals dicarbonyl substances such as glyoxal and methylglyoxal (in the case of Fru derivatives and G-3-P) were found. It should be mentioned that in free radical transformations of Fru-1,6-P2 initiated by Fe2+/t-BuOOH the ratio between the amount of methylglyoxal and that of inorganic phosphate appeared to be by ~ 30 times higher than in the case of g-irradiation of the appropriate solutions (Figure 5A and Figure 5B).
Figure 5.The ratio of the yields of Fru-1,6-P2 transformation products in case of tert-butyl hydroperoxide initiation (A) vs radiation initiation (B) in aqueous 0.01 M deaerated solutions.
The resultant products obtained in the experiment can arise from the generation of carbon- and oxygen-centered radicals in the initial compounds which appeared after the interaction of the initial molecules with •OH and t-BuO•. High yields of inorganic phosphate (Table 1, Figure 5A) allow us to suggest that the mechanisms of free radical transformation of Fru-1,6-P2 include two consecutive reactions of dephosphorylation of the initial molecule, as it is shown in Figure 6A. The formation of inorganic phosphate and methylglyoxal can also result from the sequence of reactions illustrated in Figure 6B.
Figure 6.Proposed reaction mechanisms of Fru-1,6-P2 transformations under the action of hydroxyl and alkoxyl radicals.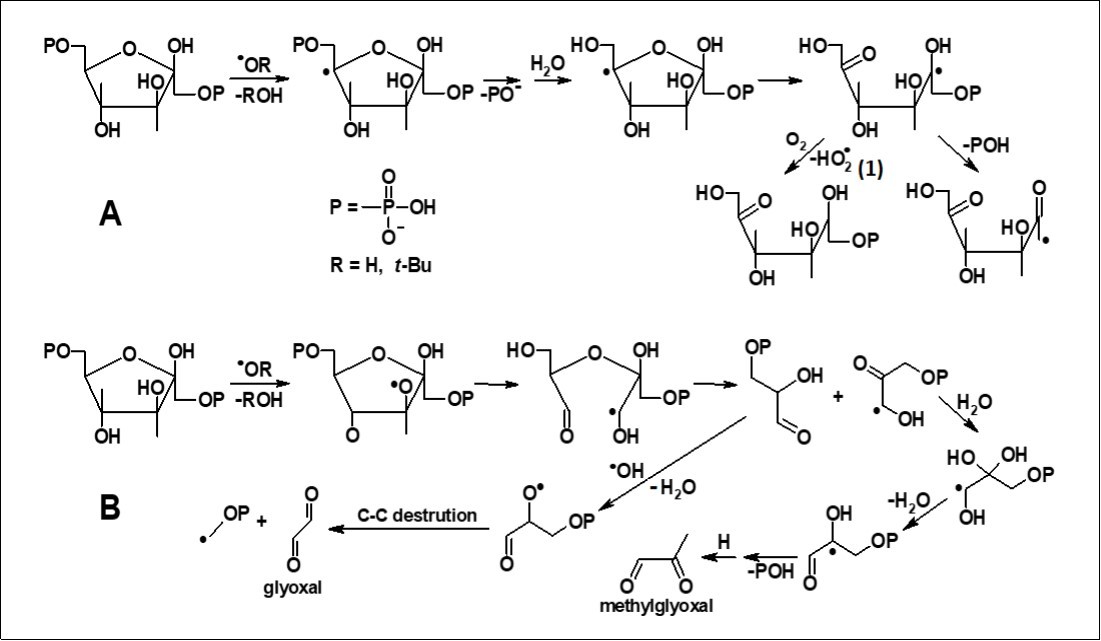
In systems containing oxygen the radiation-chemical yield of inorganic phosphate from Fru-1,6-P2 appearedto decrease by approximately 40% as compared to the argon-saturated systems (Table 1); while in oxygen-saturated systems glyoxal and methylglyoxal did not accumulate at all (Table 1). These experimental data can be account for the ability of molecular oxygen 29 to oxidize carbon-centered radicals at the α-position to hydroxyl groups, thereby blocking their further transformations. Figure 6A shows a similar reaction 1 between oxygen and a free radical intermediate of Fru-1,6-P2 degradation.
Formation of Superoxide and Organic Free Radicals in the Reaction of Methylglyoxal with Lysine.
According to the pertinent literature on the subject, α-oxoaldehyde such as methylglyoxal plays an important role in the non-enzymatic and enzymatic production of free radicals in biological systems 5, 17, 20, 30, 31, 32. For example, the free radical derivatives can be formed in the reaction of methylglyoxal-induced protein glycation 18. Since lysine elements are known to be one of the major targets for reactive carbonyl compounds in proteins 10; we studied the interaction of this amino acid with methylglyoxal.
The application of chemiluminescence revealed that the superoxide anion radical gets formed in the mixture of methylglyoxal with L-lysine at pH 7.8 (Figure 7). Lucigenin-dependent chemiluminescence was measured in the reaction mixture that contained the following elements: K,Na-phosphate buffer (0.1 M, pH 7.8), 20 μM lucigenin, 0.015 M methylglyoxal, 0.015 M L-lysine (upper curve); the same + SOD 120 units (lower curve). In this figure the insert stands for the kinetics of formazane formation during the reaction of methylglyoxal with L-lysine. The reaction showed by the mixture ran as follows: carbonate buffer (0.1 M, pH 9.5), 10 mM methylglyoxal and 10 mM L-lysine (upper curve); the same + SOD 120 units (lower curve).
SOD under these conditions almost completely inhibits the chemiluminescence of lucigenin, which proves some dependence between this process and the presence of О2·- (Figure 7, lower curve). We also assessed the superoxide anion production through the accumulation of formazan on NBT reduction in carbonate buffer, pH 9.5. (Inset, Figure 7). The accumulation of formazan under these conditions might not depend on О2·-, since NBT may probably be reduced by other intermediates of methylglyoxal reaction with L-lysine. Nevertheless, judging by the ability of SOD to inhibit significantly (more than 4 times) the formation of formazan under the above-mentioned conditions, we may conclude that the better part of NBT is reduced owing to the action of О2·- (Inset, Figure 7).
Figure 7.Production of superoxide anion radical in the reaction of methylglyoxal with L-lysine.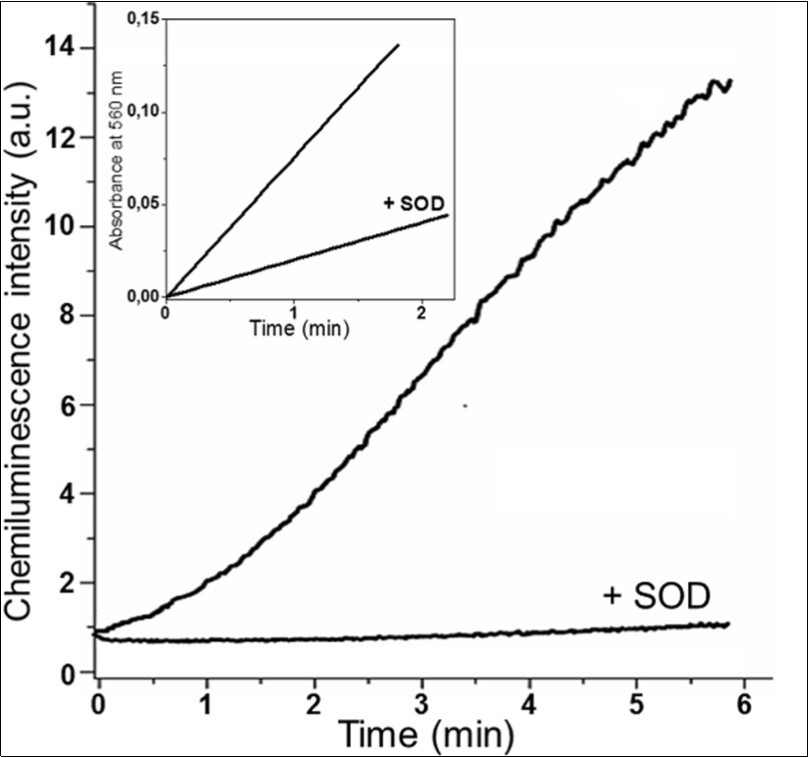
Thus, the functioning of superoxide dismutase (SOD) effect has been revealed. Superoxide-depended chemiluminescence of lucigenin in the reaction mixture contained: K,Na-phosphate buffer (0.1 M, pH 7.8), 20 μM lucigenin, 0.015 M methylglyoxal, 0.015 M L-lysine (upper curve); the same + SOD 120 units (lower curve). Inset is the kinetics of formazan formation during the reaction of methylglyoxal with L-lysine. The reaction showed by the mixture ran as follows: carbonate buffer (0.1 M, pH 9.5), 10 mM methylglyoxal and 10 mM L-lysine (upper curve); the same + SOD 120 units (lower curve).
The effect of SOD can account for the fact that this enzyme removes superoxide formed in the tested model system. Indeed, the data obtained in the experiment 17 is the evidence that О2·- is formed by a single-electron oxygen reduction by methylglyoxal anion radical (MGO·-) in accordance with the reaction:
MGO·- + О2 → MGO + О2·- ......(2).
(Figure 8) shows the results of the EPR spectroscopic study of the products obtained in L-lysine reactions with methylglyoxal (Figure 8, spectra B and E). The data presented in this figure demonstrate that free radical intermediates are formed under anaerobic conditions in the reaction between L-lysine and methylglyoxal. EPR spectra were recorded 4 min after the components had been mixed under aeration (spectrum A and D) or under nitrogen purging (spectrum B and E); several samples have been purged with nitrogen for 10 min (hypoxic conditions), which was followed by another 4 min of incubation after the switchover to aerobic conditions (spectrum C and F). In samples D-F SOD (400 units/ml) was added.
The EPR spectrum recorded in the reaction of L-lysine with methylglyoxal has a multicomponent hyperfine structure. Previously, such EPR spectrum has been recorded in the reaction mixture containing L-alanine and methylglyoxal 17. In the present work, using C13- and N15-substituted and deuterated L-alanine derivatives it has been shown that the EPR spectrum is a superposition of signals of a methylglyoxal anion radical and a cation radical of Schiff base cross-linking (dialkylimine) that appears in the interaction between methylglyoxal and the amino acid. This statement allowed us to suggest that the EPR spectrum observed in our experiments can be also a superposition of MGO·- signals and the cation-radical of methylglyoxal dialkylimine with lysine. Noteworthy as well, only trace quantities of free radical intermediates were registered under aeration of the reaction mixture (Figure 8, spectra A and D).
In samples D-F added SOD (400 units/ml). EPR spectra were recorded 4 min after the components had been mixed under aeration (spectrum A and D) or under nitrogen purging (spectrum B and E); several samples were purged with nitrogen for 10 min (hypoxic conditions) and followed by another 4 min of incubation after the switchover to aerobic conditions (spectrum C and F). The simulation of EPR spectra of methylglyoxal anion radical (G).
The decrease in the concentration of organic free radicals recorded by EPR in aerated reaction medium is probably not associated with the inhibition of their formation. Indeed, with nitrogen purging the content of the organic free radicals, the reaction reached its maximum in 8-9 min after the reaction components had been mixed; but after gas was replaced with air the level of these free radicals in the medium dropped dramatically (Figure 8 and Figure 9).
Figure 8.EPR spectra of organic free radicals formed in the reaction of a mixture containing methylglyoxal (0.16 M) and L-lysine (0.16 M) in K,Na-phosphate buffer (0.2 M, pH 7.8) (A-F). The simulation of EPR spectra of methylglyoxal anion radical (G).
Figure 9.The effect oxygen and SOD produce on the level of organic free radicals resulting from the reaction between methylglyoxal and L-lysine. In the figure: EPR signals of these radicals recorded under nitrogen purging are put in gray; the same samples 2 and 6 min after the beginning of aeration – in white. The reaction mixture content was the same as in the legend to Fig. 3. All values are mean ± SEM. n=5; *p<0.01.
Under these experimental conditions SOD most probably would reduce the rate of the intensity of EPR signal decreasing during aeration (Figure 9). The EPR spectrum containing five components of hyperfine structure and a g-factor equal to 2.0045 were recorded during aeration of the reaction medium in the presence of SOD (Figure 8, spectrum F).
According to the data presented in the literature, the characteristics of the EPR spectrum presented in Figure 8 (spectrum F) correspond to the signal of cis-form of methylglyoxal anion radical (MGO·-) 17, 20, 33. This fact confirms the above-mentioned assumption that free radical intermediates of L-lysine reaction with methylglyoxal are MGO·- and the cation radical of dialkylimine. Thus, molecular oxygen seems to interact directly with the free radical derivatives of methylglyoxal and dialkylimine; the products formed in this reaction are not registered by EPR (Figure 8 and Figure 9). However, SOD protects the methylglyoxal anion radical under aerobic conditions, which proves the possibility of MGO·- elimination under the effect of superoxide. Indeed, it has been found out that in aqueous media О2·- reduces some organic radicals 34, 35. In particular, superoxide is an electron donor for the reduction of the protonated nitrosobenzene anion radical 36. By analogy, it can be suggested that О2·- interacts with the methylglyoxal anion radical, reducing it to non-radical products in accordance with the reaction:
MGO·- + H++ О2·- → non-radical product + О2 .....(3)
The state-of-the-arts analysis 17, 18, 30 revealed that a sequence of reactions normally result in the formation of free radicals in the interaction of amino acids with carbonyl compounds (Figure 10). The presented scheme shows that dialkylimine is a Schiff base, which is a product of interaction of methylglyoxal carbonyl groups with two L-lysine molecules. As a result of the reaction of dialkylimine with another molecule of α-oxoaldehyde, the Schiff base cation radical and MGO·- are formed, respectively (Figure 10). Such a peculiarity of the chemical structure seems to reduce the ability of dialkylimines to participate in reactions of single-electron oxidation/reduction. Since SOD less effectively prevents the dissipation of the dialkylimine cation radical than that of methylglyoxal anion radical (Figure 8 and Figure 9), it can be suggested that a free radical derivative of dialkylimineis is mainly used in the reactions with molecular oxygen. Carbon-centered free radicals of amino acids, lipids and sugars are known to interact with molecular oxygen with the formation of peroxyl radicals, which are further transformed into non-radical products 1, 2, 9, 12, 13. However, in contrast to the carbon-centered allyl radicals, a free electron in the dialkylimine cation radical is localized in the system of coupled double bonds between the Schiff base nitrogen atoms and methylglyoxal carbons (Figure 10). Meanwhile, such a system of coupled double bonds is present in the molecule of paraquat (methyl viologen). The reaction of a single-electron oxygen reduction by the paraquat cation radical normally forms a paraquat dication and a superoxide anion 1, 21. The analogous reaction between oxygen and the dialkylimine cation radical may probably occur under the conditions of our experiments.
The obtained results allow us to extend and expand the previously proposed mechanism for generating the superoxide radical during the glycation reaction of amino acids by methylglyoxal 17. The functioning of the mechanism presupposes that the reactions presented in Figure 10 result in the formation of MGO·-, which further reduces oxygen to О2·- (reaction 3). We assume that О2·- is not only formed in the reactions involving MGO·- but also seems to participate in them (Figure 10).
Figure 10.Proposed mechanisms for the formation of various products during the interaction of methylglyoxal with L-lysine.
These assumptions explain the effect of oxygen on the kinetics of accumulation and dissipation of free radicals having emerged in the mixture of L-lysine and methylglyoxal. The paper 17 has shown that accumulation of MGO·-and Schiff base cross-linked cation radical depends on the oxygen concentration only insignificantly. According to our data, this fact may be associated with the application of L-alanine as an amino acid in the cited work and by a high pH value of the reaction medium (carbonate buffer, pH 9.5). It should be pointed out that the model system proposed in the paper (phosphate buffer, pH 7.8) is much closer to physiological conditions. Thus, it is most likely that oxidative modification of proteins and other biomolecules might be the influence a local generation of superoxide produces on the interaction between the residues of L-lysine (and probably other amino acids) and α-oxoaldehydes. This phenomenon of non-fermentative superoxide generation might be an element of autocatalytic intensification of pathophysiological action of carbonyl stress.
Glycation of biopolymers by reactive carbonyl compounds (Maillard reaction) leads to the formation of the advanced glycation end products – the biomarkers of pathophysiological processes associated with diabetes and various other metabolically grounded diseases 5, 7, 10. Using different regulatory pathways, these products can provoke NADPH oxidase and mitochondria to form a superoxide radical 3, 24, 37. Oxidative stress in diabetes mellitus can also be triggered by inhibition of antioxidant enzymes, which, in its turn, was caused by reactive dicarbonyl compounds 15, 38. At the same time, as it has been noted above, the non-enzymatic reactions may get involved in the formation of О2·- under the carbonyl stress.
Recent studies have shown that glucose oxidation by a hydroxyl radical leads to the formation of a-oxoaldehyde as 3-deoxyglucosone 39, that agrees with the proposed mechanism of non-enzymatic synthesis of methylglyoxal. The data obtained in our study also highlight the important role the free radical reactions play in non-enzymatic transformations of sugars and other carbonyl compounds. It should be also noted that the synthesis of methylglyoxal from hexose phosphates in the presence of reactive oxygen and organic free radicals and provokes the consequent formation of О2·- in modifications of amino acids by methylglyoxal. Thus, under the conditions of hyperglycemia the oxidative stress can cause non-enzymatic accumulation of methylglyoxal, which, in its turn, can further stimulate oxidative stress as well. Therefore, the concepts of ‘oxidative stress’ and ‘carbonyl stress’ should be considered rather controversial, since various stages of these processes typical of these stresses not infrequently merge and converge into each other. These data may allow for proposing that molecular mechanisms provoking the diseases associated with oxidative and carbonyl stress (such as atherosclerosis and diabetes) should be more complicated than they used to be thought of and are to be studies with close attention to the interplay of reactive carbonyl compounds and ROS.
Conclusions
The obtained data should be taken into account when describing the free radicals giving rise to the formation of dicarbonyls in pathophysiological processes involving Fru-1,6-P2 and other Glc metabolites. At the same time, the toxicity of α-oxoaldehydes can be associated with further free radical reactions. Indeed, methylglyoxal is not only a product of free radical processes, but also plays an important role in their development.
Acknowledgements
This work is partially supported by grants from the Russian Foundation for Basic Research (18-015-00125 and 19-015-00444). We thank prof. Ruuge E.K. for a valuable discussion of the manuscript and consultation given on EPR spectroscopy. We express our gratitude to Rosetta stone MSU (Sharapkova A. аnd Sapunova O.) for a careful reading of the manuscript.
References
- 1.Halliwell B, JMC Gutteridge. (1999) Free radicals in biology and medicine. In:. Free Radicals in Biology and B Halliwell, JMC Gutteridge, (eds.) , Oxford 1-25.
- 3.A1 Tarafdar, Pula G. (2018) . The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders.,Int J Mol Sci19(12) 3824.
- 4.Lankin V Z, Tikhaze A K, Konovalova G G, Kumskova E M, Shumaev K B. (2010) Aldehyde-dependent modification of low density lipoproteins. In: Handbook of Lipoprotein Research.NY. Nova Sci85-107
- 5.Nowotny K, Jung T, Höhn A, Weber D, Grune T. (2015) . Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus.,Biomolecules5: 194-222.
- 6.Lankin V Z, Tikhaze A K. (2017) . Role of Oxidative Stress in the Genesis of Atherosclerosis and Diabetes Mellitus: A Personal Look Back on 50 Years of Research.,СurrАgingSci10: 18-25.
- 7.Liguori I, Russo G, Curcio F, Bulli G, Aran L et al. (2018) Oxidative stress, aging, and diseases.,ClinIntervAging13:. 757-772.
- 8.Lankin V Z, Tikhaze A K. (2003) Free radical lipoperoxidation during atherosclerosis and antioxidative therapy of this disease. In:. A Tomasi et al.(eds.).Free Radicals, Nitric Oxide and Inflammation: Molecular, Biochemical and Clinical Aspects.Amsterdam, etc.: IOS Press, NATO Science Series,344: 218-231.
- 9.GuéraudF AtalayM, BresgenN CipakA, EcklPM HucL, JouaninI SiemsW, Uchida K. (2010) Chemistry and biochemistry of lipid peroxidation products.,FreeRadicRes. 44, 1098-124.
- 10.Vistoli G, D, Cipak A, Zarkovic N, Carini M et al. (2013) Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation.,FreeRadicRes47:. 3-27.
- 11.Jahn M, Spiteller G. (1996) Oxidation of D-(-)-ribose with H2O2and lipid hydroperoxides.,J. Biosciences,5: 870-876.
- 12.Spiteller G. (2005) The relation of lipid peroxidation processes with atherogenesis: A newtheory on atherogenesis.,MolNutrFood. Res,49: 999-1013.
- 13.Spiteller G. (2008) Peroxyl Radicals Are Essential Reagents. in the Oxidation Steps of the Maillard Reaction Leading to Generation of Advanced Glycation End Products.,Ann NYAcadSci,1126: 128-133.
- 14.Lankin V, Konovalova G, Tikhaze A, Shumaev K, Kumskova E et al. (2014) The initiation of free radical peroxidation of low-density lipoproteins by glucose and its metabolite methylglyoxal: a common molecular mechanism of vascular wall injure in atherosclerosis and diabetes.,Mol. CellBiochem,395: 241-52.
- 15.Lankin V Z, Konovalova G G, Tikhaze A K, Shumaev K B, Belova-Kumskova E M et al. (2016) Aldehyde inhibition of antioxidant enzymes in blood of diabetic patients.,J. Diabetes,8: 398-404.
- 16.Lankin V Z, Tikhaze A K, Kumskova E M. (2012) Macrophages actively accumulate malonyldialdehyde-modified but not enzymatically oxidized low density lipoprotein.,Mol. CellBiochem,365: 93-98.
- 17.Yim H, Kang S O, YCh Hah, Chock P B, Yim M B. (1995) Free radicals generated during the glycation reaction of amino acids by methylglyoxal: A model study of protein-cross-linked free radicals.,J Biol Chem,270:. 28228-28233.
- 18.YimMB YimHS, Lee C, Kang S O, Chock P B. (2001) Protein glycation: creation of catalytic sites for free radical generation.,Ann N YAcadSci 928:. 48-53.
- 19.Fricke H, Hart E J. (1966) Chemical dosimetry. Radiation Dosimetry.Academic press In: FH Attix, WC Roesch (eds.) , New York167–177 .
- 20.Thornalley P J. (1985) Monosaccharide autoxidation in health and disease.,Environ. HealthPerspect,64: 297-307.
- 21.Mason R P. (1990) Redox Cycling of Radical Anion. Metabolites of Toxic Chemicals and Drugs and the Marcus Theory of Electron Transfer Environ.,HealthPerspect87: 237-243.
- 22.Latunde-Dada G O. (2017) Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy.,BiochimBiophysActa Gen Subj. 1861, 1893-1900.
- 24.Stefano G B, Challenger S, Kream R M. (2016) Hyperglycemiaassociated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders.,Eur JNutr,55:. 2339-2345.
- 25.Shadyro O I. (1997) Radiation-induced free radical fragmentation of cell membrane components and the respective model compounds. In:. Free Radicals in Biology and Environment.Kluwer F Minisci (ed.) , Netherlands317–329 .
- 26.Edimecheva I P, Kisel R M, Shadyro O I, Kazem K, Murase H et al. (2005) Homolytic cleavage of the O-glyсoside bond in carbohydrates: a steady-state radiolysis study.,JRadiatRes46(3). 319-324.
- 27.Lisovskaya A G, Sladkova A A, Sosnovskaya A A, Shadyro O I. (2012) Reactions of aminyl radicals during radiolysis and photolysis of aqueous solutions of amino alcohols and their derivatives.,HighEnergChem46(4). 241-246.
- 28.Hawkins C L, Davies M J. (2001) Generation and propagation of radical reactions on proteins.,BiochimicaetBiophysicaActa1504(2‒3). 196-219.
- 29.Shadyro O I, Sosnovskaya A A, Vrublevskaya O N. (2003) C-N bond cleavage reactions on the radiolysis of amino-containing organic compounds and their derivatives in aqueous solutions.,Int. 79(4), 269-279.
- 30.Suji G, Sivakami S. (2007) DNA damage during glycation of lysine by methylglyoxal: assessment of vitamins in preventing damage.,Amino. Acids,33: 615-621.
- 31.Kalapos M P, Desai K M, Wu L. (2010) Methylglyoxal, oxidative stress, and aging. In:. S Bondy, K Maiese(eds.).Oxidative Stress in Applied Basic Research and Clinical Practice: Aging and Age-Related Disorders.Springer,NewYork149-167 .
- 32.Kosmachevskaya O V, Shumaev K B, Nasybullina E I, Topunov A F. (2014) . Formation of Nitri- and Nitrosylhemoglobin in systems modeling the Maillard reaction.,Clinical Chemistry & Laboratory Medicine52(1) 161-168.
- 33.PRC Gascoyne, MCR Symons, McLaughlin J A. (1983) Spontaneous electron transfer in the reaction between methylglyoxal and methylamine.Int J Quant Chem: Quant BiolSymp10:. 123-132.
- 34.Bisby R H, Paker A W. (1991) Reactions of the alpha-tocopheroxyl radical in micellar solutions studied by nanosecond laser flash photolysis.,FEBS. Lett,290: 205-208.
- 35.D’Alessandro N, Bianchi G, XJF Fang, Schuchmann H P, von Sontag CJ. (2000) Reaction of superoxide with phenoxyl-type radicals.,Journal of the Chemical Society Perkin Transactions. 22(9), 1862-1867.
- 36.Todres Z V. (2003) Organic Radical Ions: Chemistry and Applications., Marcel Dekker,New York1-417.
- 37.Zhou X, Weng J, Xu J, Xu Q, Wang W et al. (2018) . Mdia1 is Crucial for Advanced Glycation End Product-Induced Endothelial Hyperpermeability.,CellPhysiolBiochem45(4) 1717-1730.
Cited by (11)
- 1.Tikhaze A. K., Domogatsky S. P., Lankin V. Z., 2021, Clearance of Carbonyl-Modified Low-Density Lipoproteins in Rabbits, Biochemistry (Moscow), Supplement Series B: Biomedical Chemistry, 15(2), 119, 10.1134/S1990750821020104
- 2.Tikhaze A.K., Domogatsky S.P., Lankin V.Z., 2020, Elimination kinetics of carbonyl-modified low density lipoproteins from bloodstream, Biomeditsinskaya Khimiya, 66(6), 437, 10.18097/pbmc20206606437
- 3.Lankin Vadim Z., Sharapov Mars G., Tikhaze Alla K., Goncharov Ruslan G., Antonova Olga A., et al, 2023, Dicarbonyl-Modified Low-Density Lipoproteins Are Key Inducers of LOX-1 and NOX1 Gene Expression in the Cultured Human Umbilical Vein Endotheliocytes, Biochemistry (Moscow), 88(12-13), 2125, 10.1134/S0006297923120143
- 4.Lankin V. Z, Sharapov M. G, Tihaze A. K, Goncharov R. G, Antonova O. A, et al, 2023, Dicarbonyl-modified low-density lipoproteins are key inducers of LOX-1 and NOX1 gene expression in cultured human umbilical vein endotheliocytes, Биохимия, 88(12), 2517, 10.31857/S0320972523120138
- 5.Lankin Vadim Z., Tikhaze Alla K., Kosach Valeria Ya., 2022, Comparative Susceptibility to Oxidation of Different Classes of Blood Plasma Lipoproteins, Biochemistry (Moscow), 87(11), 1335, 10.1134/S0006297922110128
- 6.Shumaev K.B., Kosmachevskaya O.V., Grachev D.I., Timoshin A.A., Topunov A.F., et al, 2021, Possible mechanism of antioxidant action of dinitrosyl iron complexes, Biomeditsinskaya Khimiya, 67(2), 162, 10.18097/pbmc20216702162
- 7.Шарапов М.Г., Гудков С.В., Ланкин В.З., Новоселов В.И., 2021, Роль глутатионпероксидаз и пероксиредоксинов при свободнорадикальных патологиях, Биохимия, 86(11), 1635, 10.31857/S0320972521110038
- 8.Lankin V. Z., Tikhaze A. K., Kosach V. Ya., Konovalova G. G., Kudryashova A. V., 2023, Modification of low-density lipoproteins by low molecular weight carbonyl products of free-radical oxidation of lipids and carbohydrates plays a key role in atherosclerotic lesion of the vascular wall and in endothelial dysfunction, Acta Biomedica Scientifica, 8(3), 14, 10.29413/ABS.2023-8.3.2
- 9.Sharapov Mars G., Gudkov Sergey V., Lankin Vadim Z., Novoselov Vladimir I., 2021, Role of Glutathione Peroxidases and Peroxiredoxins in Free Radical-Induced Pathologies, Biochemistry (Moscow), 86(11), 1418, 10.1134/S0006297921110067
- 10.Shumaev K. B., Kosmachevskaya O. V., Grachev D. I., Timoshin A. A., Topunov A. F., et al, 2021, A Possible Mechanism of the Antioxidant Action of Dinitrosyl Iron Complexes, Biochemistry (Moscow), Supplement Series B: Biomedical Chemistry, 15(4), 313, 10.1134/S1990750821040090
- 11.Lankin Vadim Z., Tikhaze Alla K., Sharapov Mars G., Konovalova Galina G., 2024, The Role of Natural Low Molecular Weight Dicarbonyls in Atherogenesis and Diabetogenesis, Reviews in Cardiovascular Medicine, 25(8), 10.31083/j.rcm2508295
