Pain Perception Modulates Synaptic and Somatic Plasticity in the Dentate Gyrus of Rats
Abstract
An experimental study examines how pain perception alters synaptic and somatic plasticity in the dentate gyrus. It summarizes methods, key electrophysiological findings, and implications for pain‑related cognitive effects.
Author Contributions
Academic Editor: Peter Awhen, University of Calabar, PMB 1115, Etagbor, Cross River State, Nigeria
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2024 Soley Arslan, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Abstract
Background
Despite extensive research on the link between pain experience and neural processes, the influences of intermittent sub-chronic pain on the hippocampal plasticity are less clear. In this work, we investigated at long-term plastic alterations in synaptic connections and neuronal function in rats with dentin erosion.
Methods
Experiments were carried out on control and the rats with dentin erosion. The dentin hypersensitivity test was used to assess the behavioral reaction of rats by injecting of cold or warmish water. The short-term and the long-term plasticity of the dentate gyrus area were induced by electrical stimulation of the perforant pathway. The expressions of certain neurodegeneration-related genes were assessed by quantitative real-time PCR. Western blot was used to measure the expression of key proteins associated with neuronal plasticity.
Results
Exposure of teeth to acetic solution caused molar tooth lesions with increased motor reaction when stimulated with cold water. These rats expressed facilitated short-term synaptic depression, post-tetanic potentiation and long-term potentiation. The PSEN2-mRNA levels significantly elevated in the rats exposed to painful stimuli, compared to the rats exposed to painless stimuli. There was no significant difference in MAPT-mRNA, JNK-mRNA, and BACE1-mRNA. Pain perception prevented down regulation of ERK1/2 phosphorylation by dentin erosion.
Discussion
The present study suggests a link between peripheral nociceptive information and neuronal plasticity in the dentate gyrus. The simultaneous presence of increased PSEN2 expression and facilitated plasticity in pain-perceived rats requires further investigation into the role of pain in the development of neurodegenerative alterations.
Introduction
Synaptic plasticity, or the alteration of synaptic strength, is assumed to be a cellular mechanism of learning and memory processes. Postsynaptic Calcium (Ca) entry, depending on current dynamics, activates intracellular kinase or phosphatase cascades that result in AMPA receptor insertion or removal from the membrane. This, in turn, raises post-synaptic NMDA excitation in response to a given pre-synaptic stimulus. As a consequence, the enlargement of existing synapses, the development of new synapses, and the loss of inappropriate synaptic connections reconfigure the neural network for information processing 16, 22.
Experimentally, different patterns of electrical stimulation that are relatively confined to the perforant pathway produces synchronized activity of many granule cells in the dentate gyrus which can be extracellularly recorded as a field potential (FP) by a glass microelectrode. The FP consists of a positive-going synaptic component (field excitatory postsynaptic potential, fEPSP), with a superimposed non-synaptic component (population spike, PS). The fEPSP is a measure of postsynaptic depolarization which makes the post synaptic neuron more likely to fire an action potential. The PS reflects the somatic response, the amplitude of which is dependent on the number of granule cells that discharge in synchrony. Both components of the field potential can be subjected to a relatively permanent change independently of each other. Long-term potentiation (LTP) is characterized by an increase in neuron output with or without changes in synaptic plasticity following a few trains of high-frequency stimulation.
Recent work has recognized that the LTP at both excitatory and inhibitory synapses can function as a prime mechanism underlying pathological pain as well 32, 26. A growing body of data from behavioral, electrophysiological, molecular/biochemical, and functional imaging research supports the hypothesis of a link between hippocampal formation and pain perception. LTP of CA1 neurons in mice, for instance, was enhanced after tail amputation 45. Subcutaneous injection of whole bee venom solution also significantly enhanced the induction probability and the magnitude of LTP elicited in both the dentate gyrus and CA1 area when compared to the control 50. However, there are also studies whose results are inconsistent with these studies: Chronic neuropathic pain causes memory deficits in rodents 36, induces LTP at C-fiber synapses in the spinal dorsal horn 49, but impairs LTP at CA3–CA1 synapses in the hippocampus 36. The inconsistent findings of all these studies show that there is a continuing need for further research to understand the relationship between pain and plasticity.
The present study investigates the modulation of neuronal plasticity in the dentate gyrus with chronic and intermittent pain which is induced by dentin hypersensivity in rats with dental erosion. The presence of two distinct afferent pathways that carry peripheral nociceptive information to DG granular cells is the primary source of the assumption of a causal relationship between pain perception and the hippocampus 31. One of these afferents is cholinergic projection pathway which originates from medial septal nucleus. The other is the glutamatergic perforant pathway, which transmits nociceptive information from the primary somatosensory cortex to posterior parietal association cortex, and the entorhinal cortex and then to the dentate gyrus 15. Previous studies have reported findings indicating a role for the mitogen-activated protein kinase (MAPK) signaling pathways in the modulation of LTP by pain in dorsal horn neurons. For instance, following painful stimulation, dorsal horn neurons express an LTP that is blocked by MAPK kinases (MEK) inhibitors 37, 47. Activity-dependent insertion of AMPA receptor requires pain-induced activation of the MEK/ERK pathway in dorsal horn neurons 18. In addition, a specific role of MAPKs has also been suggested for the development of pain hypersensitivity following tissue and nerve injury (reviewed in 23). The MAPK signaling pathways control gene expression in a number of ways including the phosphorylation and regulation of transcription factors, co-regulatory proteins and chromatin proteins 46. We, therefore, hypothesized that pain modulates neuronal plasticity in the dentate gyrus through the MAPK signaling system and investigated whether this modulation causes transcriptional changes in some neurodegenerative proteins. To do this, we studied an experimental model of denti hypersensitivity (DH), a most common source of pain in humans.
Methods
The experimental setup of this study includes electrophysiological and molecular approaches used to demonstrate that modulation of neuronal plasticity by pain sensation is accompanied by changes in the hippocampal expression of some neurodegenerative proteins. To represent a common pain pattern in humans, the dentin hypersensitivity model was chosen, which begins suddenly, causes periods of discomfort, and persists throughout the time of contact with the stimulus 2, 40, 20. Induction of neuronal plasticity was achieved by stimulating the perforant pathway with high and low frequency stimulation patterns. Western blot technique was used to demonstrate the contribution of three MAPK family members, which are major regulators of the transcriptional response, in the modulation of synaptic plasticity by pain sensation 46. Quantitative mRNA measurements of MAPT, JNK, PSEN2 and BACE1 genes were used to demonstrate that modulation of neuronal plasticity by pain may cause transcriptional changes in neurodegenerative proteins.
Experimental design
Thirty-nine male Wistar rats were divided into two groups, based on dentin erosion treatment. The rats in the control group (n = 13) were kept in standard conditions and housed in regular cages in the same room as the rats with dentin erosion. The remaining rats were allocated to the experimental groups in which dentin erosion was produced in rats by drinking an isotonic solution (Powerade®, lemon flavor, pH 2.7) as drinking water for 42 days to rats. During the last 7 days, these rats were exposed to dentin hypersensitivity test by injecting of cold (4 °C, painful group, Group E1, n = 13) or warmish (16 oC, painless group, Group E0, n = 13) water. Injections were applied by an insulin-size syringe containing a silicon cannula for 5 seconds. During hypersensivity test, two observers scored behavior response independently as follows: 0 = no response, 0.5 = slight contraction of the body, 1 = contraction of the body, 2 = strong contraction of the body with a short vocalization; and 3 = strong contraction of the body with a prolonged vocalization 4. One day after hypersensitivity tests, the jaws were removed for scanning electron microscopy (SEM) following electrophysiology.
Recording of Field Potentials
Hippocampal field potentials were recorded from anesthetized rats as previously described 43. At the beginning of experiment, the input/output curve was obtained by plotting the raw values of fEPSP slope and PS amplitude against electrical pulse intensities varying from 0.1 mA to 1.5mA. For this, the PP was stimulated at a frequency of 0.05 Hz with 0.2 mA steps after 15minutes of stable baseline recordings. The pulse intensity that produces a half of the maximum PS amplitude (test stimulus) was determined to be used throughout the experiment. Following I/O curve protocol, electrical stimulation of the PP continued for 75 minutes at a frequency of 0.33 Hz (one pulse every 30 second). After 15-min baseline recording, high-frequency stimulation (HFS) was delivered four times with 5-min intervals at 100 Hz, bipolar, biphasic, square wave with a width of 125 μsec, or low-frequency stimulation (LFS) at 1 Hz. Immediately after completion of electrophysiology, in deeply anesthetized rat, the brain was carefully dissected out from the skull, placed into ice-cold phosphate-buffered saline, cut along the longitudinal fissure of the cerebrum using a surgical knife, and cut off the regions posterior to lambda (midbrain, hindbrain, and cerebellum). Then medial side of the cerebral hemisphere was placed up and carefully removed the diencephalon (thalamus and hypothalamus) under a dissection microscope, allowing for visualization of the hippocampus. Using a forceps, right and left hippocampus separately dissected from surrounding area and weighed.
Western Blotting
The whole hippocampus was lysed in RIPA buffer (sc-24,948; Santa Cruz Biotechnology, Santa Cruz, California, USA) with sonication. The homogenates were centrifuged at 15,000 ×g for 20 min at 4°C. Forty micrograms of total protein from each sample were separated on a 10% of SDS-polyacrylamide gel and transblotted onto a PVDF membrane (GE Healthcare, 10,600,021, Amersham Hybond). The membranes were blocked with 5% of non-fat dry milk in Tris-Buffered Saline (TBS) containing 0.1% of Tween-20 (TBS-T) for 1 hour at room temperature before being incubated with primary antibodies overnight at 4°C (16, 17). The following primary antibodies were used: ERK1/2 (#4695, 1:1000, Cell Signaling), p-ERK1/2 (#9101; 1:1000, Cell Signaling), JNK (#9252, 1:1000, Cell Signaling), p-JNK (#9251; 1:1000, Cell Signaling), P38 (#9212; 1:1000, Cell Signaling), p-P38 MAPK (#9211, 1:1000, Cell Signaling). The membranes were then washed briefly with TBS-T and incubated for 1 hr at room temperature in a 1:4,000 dilution of goat anti-rabbit IgG (sc2004; Santa Cruz Biotechnology) or goat anti-mouse IgG (sc-2005; Santa Cruz Biotechnology) secondary antibody conjugated to horse radish peroxidase. The bound antibodies were visualized by the G: Box Syngene System (Beacon House, Cambridge, UK) using an enhanced chemiluminescence substrate (Pierce™ ECL Western Blotting Substrate, catalog number: 32,106) according to the manufacturer's instructions. The blots were subsequently stripped and reprobed with a β-actin mouse monoclonal antibody (AC-15, sc-69,879, Santa Cruz Biotechnology) to confirm the equal loading of the protein samples in the gel. The optical density (OD) of the blots was measured with ImageJ software (National Institutes of Health, USA). The OD of each band was normalized to the corresponding β-actin band. Relative OD (ROD) was calculated dividing the optical density of the analyzed sample by the first band of each blot. The OD of a protein band in a sample was expressed as relative OD (ROD) according to the amount of protein loaded into the corresponding well. Each gel or blot was duplicated. Data were analyzed using Kruskal–Wallis and Mann–Whitney U test.
Real-time Quantitative Polymerase Chain Reaction (RTqPCR) Analysis
The RT-qPCR was performed using a CFX Connect Real-Time PCR detection System (BioRad) with SsOAdvanced Universal IT SYBR Green Supermix (BioRad). Primer sets used were validated by and purchased from Oligomer and Sentebiolab. The primer sequences used are given in Table 1. The cycle threshold (Ct) was determined for each target gene in duplicate. The β-Actin gene was used as the house- keeping gene (reference gene). At the end of the process, Ct (threshold cycle) values was recorded. The Ct values obtained were calculated and normalized using the 2−△△Ct method.
Table 1. Primer sequences of mRNA used for quantitative Reverse Transcription PCR (q-rtPCR).| Primer | Sequences (5 ı –3 ı ) | Tm ( o C) | Supplier | Dilution |
| MAPT - F | 5I-GAC TCC TCC AGG ATC AGG TG-3I | 61 | Oligomer | 1:10 |
| MAPT - R | 5I-GGT AGG GAT GGG GTA CGG-3I | 61 | Oligomer | 1:10 |
| JNK - F | 5I-GAA TGT CCT ACC TTC TCT CTA TCA AAT GC-3I | 62 | Oligomer | 1:10 |
| JNK - R | 5I-TCC TTG CCA GTC CAA AAT CA-3I | 55 | Oligomer | 1:10 |
| PSEN2- F | 5I-AGG ACC CTA AGG CTT GCA CCT-3I | 55 | IDT | 1:10 |
| PSEN2 - R | 5I-CTA GGG AAA ATG GAA AGA GT -3I | 55 | IDT | 1:10 |
| BACE1 - F | 5I-GTG GTT ACC TTG CTG CCA TC-3I | 55 | IDT | 1:10 |
| BACE1 -R | 5I-GTG GTT ACC TTG CTG CCA TC-3I | 55 | IDT | 1:10 |
Scanning Electron Microscopy (SEM)
After euthanasia, jaws were removed, cleaned free of soft tissues, and molars was isolated for SEM analysis. The molars were submerged in acryl so that the buccal and lingual surfaces could be seen. Then the pieces underwent a dehydration process with an ascending series of acetone and weresubsequently coated with gold palladium at a thickness of approximately 15 nm (Sputter Coater MED-020, Bal-Tec). After these procedures, the specimens were observed under SEM (JEOL, JSM-6510, USA), in order to concomitantly analyze structural condition of dentin.
Data Analysis and Statistics
The slope of the fEPSP was calculated as the amplitude change at 20–80% of the voltage difference between the start and the peak of the waveform, whereas the amplitude of PS was calculated as the difference from the first positive peak to the negative peak for each field potential. The raw values of the EPSP slope and PS amplitude during the I/O experiment were analyzed using a separate two-way repeated measures ANOVA, with group (control, E0 and E1 groups) as between subjects variables, and stimulus intensity (8 levels of intensity).
Raw values of each fEPSP slope and PS amplitude were expressed as a percentage of own mean value averaged in baseline, and their means at 0-5 minutes (short-term plasticity) after induction and the last 5 minutes of recording (long-term plasticity) were calculated. Synaptic or somatic LTP was considered to be induced if the mean fEPSP slope or mean PS amplitude was greater than 120% of the baseline value 27, and if it was less than 80% to be induced LTD. Possible differences in plasticity among three groups (control, E0 and E1 groups) was analyzed separate one-way ANOVA.
Optical density of the blots was measured with ImageJ software (National Institutes of Health, USA), was normalized to the expression levels of β-actin and was expressed as an arbitrary unit relative to a sample of control group in each membrane. Kruskal-Wallis test followed by Mann–Whitney U tests was used to compare differences between groups.
A p level of ≤ 0.05 (two-tailed) was applied for all comparisons to determine statistical significance. All statistical analyses were performed using SPSS software (SPSS, Chicago, IL).
Results
Verification of pain induced by dental erosion.
A one sample SEM picture from each group can be shown in Figure 1. SEM of dental elements showed the exposed tubules in E0 and E1 groups while no tubules exposure were observed in the control groups (Figure 1). There was higher dentin hypersensivity scores in rats with dentin erosion exposed to cold water (0.538±0.144) than warmish (0.154±0.087; Z= 2.169, p = 0.030, the Mann-Whitney test), confirming thatcold stimulation increased the pain response Figure 1.
Figure 1.Jet injection of cold water to erosive molar triggers pain-related behavioral response in rats. A: Representative images of samples (SEM) with normal (top, control rat) and severe dentin erosion (middle and bottom). B: Motor reaction scores of rats with dentin erosion to warmish water (E0, n = 13) and cold water (Group E1, n = 13).
Electrophysiology
Figure 2 shows pain modulation of neuronal plasticity induced by low-frequency stimulation and high-frequency stimulation.
Figure 2.Pain modulation of neuronal long-term potentiation induced by low-frequency stimulation and high-frequency stimulation. Left Panel: The perforant pathway of control rats (black circle) and rats with dentin erosion (empty circle and grey circle: erosion with or without painful stimulus, respectively) was stimulated by electrical pulses at 1Hz for 15 min (0-15 min). It is noteworthy that the field potentials of rats with dental erosion followed the stimulation with a smaller slope and smaller amplitude compared to the control group. Right Panel: The perforant pathway of control rats and rats with dentin erosion was stimulated by electrical pulses at 100Hz four times for 15 min (0-15 min). It is noteworthy that the field potentials of rats with dental erosion followed the stimulation with higher amplitude compared to the control group when pain sensation is evoked.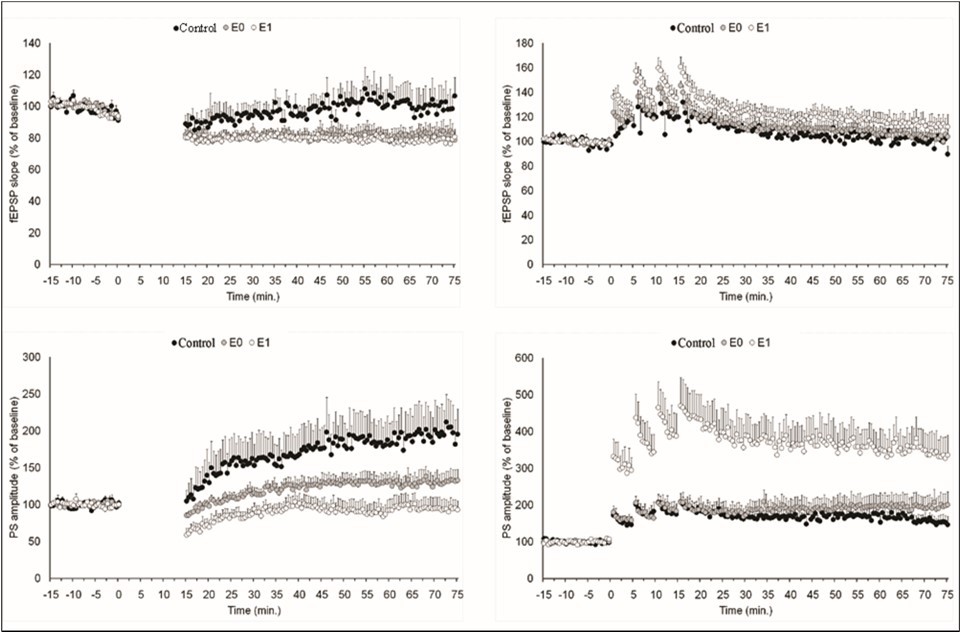
Short-Term Plasticity
The mean fEPSP slope and mean PS amplitude recorded 0-5 minutes after induction were used to assess short-term synaptic and somatic plasticity, respectively (Figure 3). LFS to the perforant pathway induced comparable early depression of synaptic strength in the control (as shown by fEPSP slope of 88.2±6.0% of baseline), E0 (81.6±2.7%) and E1 groups (79.4±2.8%, F2,15=1.227; p =0.321). The slope of EPSP following HFS, on the other hand, increased by 112.3±10.6% of baseline in the control group, 119.2±7.9% of baseline in the painless group, and 131.6±5.8% of baseline in the painful group (F2,15=1.378; p=0.277). Despite of the lack of a significant difference among groups these results indicate that both short-term synaptic depression induced by LFS and short-term synaptic potentiation induced by HFS are amplified by pain sensation.
Figure 3.Pain modulation of short-term synaptic (fEPSP slope) and somatic (PS amplitude) neuronal plasticity. The percent change in fEPSP slope or PS amplitude evaluated 55-60 min after low-frequency stimulation (LFS) or high-frequency stimulation (HFS) over baseline is defined as short term plasticity. Horizontal lines represent threshold for potentiation (>120%) and depression (<80%) of plasticity. Note that when exposed to painful stimuli, rats with dentin erosion exhibit more depressed long-term plasticity to LFS and more enhanced long-term plasticity to HFS than control rats (black bars). Grey bars: rats with dentin erosion exposed to painless stimuli. Empty bars: rats with dentin erosion exposed to painful stimuli. * indicates significant difference from control rats.
Contrary to expected, an early potentiation of the PS amplitude was observed following both LFS (121.2±25.2% of baseline) and HFS (193.9±20.2% of baseline) in control rats. The PS amplitude decreased by 67.8±8.1% of baseline after LFS but increased by 448.0±70.1% of baseline after HFS in the painful group. The PS amplitude in the painless group did not show any significant difference to pre-induction after LFS (94.3±3.2 of baseline), but potentiated by 195.0±23.4% of baseline after HFS. After a one-way ANOVA, the PS amplitude was revealed to have a significant group difference (F2,18=10.929; p=0.001), however there was a tendency for significance after LFS (F2,15=3.006; p=0.080). Tamhane's post-hoc test confirms that painful stimulation facilitates post-tetanic potentiation of the PS amplitude. (vs. control group, p=0.031; vs. painless group, p=0.031). These results indicate that short-term somatic potentiation induced by HFS is amplified by pain sensation.
Long-Term Plasticity
The mean fEPSP slope and mean PS amplitude recorded 55-60 minutes after induction were used to assess long-term synaptic and somatic plasticity, respectively (Figure. 4). In the control group, both LFS (198.8±35.9% of baseline) and HFS (154.0±16.5% of baseline) to the perforant pathway induce a long-term increase in PS amplitude although no change in fEPSP slope was observed (100.1±10.7 and 99.9±4.5 of baseline, respectively).In contrast to the control group, LFS to the perforant pathway resulted in synaptic LTD in painless group (82.5±6.0%, t5 = 0.034) and painful group (79.0±4.9, t6 = 0.008). LFS-induced long term potentiation of PS amplitude was lower in painless group (130.6±13.6%) and painful group (94.0±12.6%), but higher in painless group (197.5±31.9%) and in painful group (337.3±53.2%). One-way ANOVAs yielded significant group difference for the PS amplitude after LFS (F2,15=5.200, p=0.019) and HFS (F2,18=6.671, p=0.007).There was no significant group difference in fEPSP slope after LFS (F2,15=2.13, p=0.146) and after HFS (F2,18=1.369, p=0.281). Tamhane's post-hoc test confirms that painful stimulation facilitates long-term potentiation of the PS amplitude after HFS (p=0.038). Nevertheless, the difference between control and E1 rats showed a trend toward statistical significance after LFS (p=0.093).
Molecular Results
The biochemical conversion from short-term plasticity to LTP requires activation of intracellular kinase pathways and gene expression induced with high-frequency stimulation 14; 10. MAPK control of activity-dependent gene expression is one of the critical events for long-lasting changes at the synapse. Therefore, we looked at how HFS-induced MAPK activation and gene expression differed between rats exposed to painful and painless stimuli (Figure 4). We found that the p-ERK1/2 (ꭓ2=7.692; p=0.021) levels, but not total-ERK1/2 (ꭓ2=3.978; p=0.137), in the hippocampus differed among groups 60 minutes after HFS. Post-hoc Mann Whitney test revealed that p-ERK1/2 levels in the painless group was considerably lower than in the control group (Z=2.727, p=0.006), suggesting that pain sensation prevents down regulation of ERK1/2 phosphorylation by dentin erosion. There was no significant difference among three groups for JNK and p38-MAPK (data not shown).
Figure 4.Pain modulation of long-term synaptic (fEPSP slope) and somatic (PS amplitude) neuronal plasticity. The percent change in fEPSP slope or PS amplitude evaluated 55-60 min after low-frequency stimulation (LFS) or high-frequency stimulation (HFS) over baseline is defined as short term plasticity. Horizontal lines represent threshold for potentiation (>120%) and depression (<80%) of plasticity. Note that when exposed to painful stimuli, rats with dentin erosion exhibit more depressed long-term plasticity to LFS and more enhanced long-term plasticity to HFS than control rats (black bars). Grey bars: rats with dentin erosion exposed to painless stimuli. Empty bars: rats with dentin erosion exposed to painful stimuli. * indicates significant difference from control rats.
In addition, we found that PSEN2-mRNA levels significantly elevated in the rats exposed to painful stimuli (n=5; 1.60±0.13 fold relative to a control rat), compared to the rats exposed to painless stimuli (n=5; 0.97±0.18) in response to HFS (Z=2.104; p=0.030, Mann-Whitney U test). There was no significant difference in MAPT-mRNA (painful:1.06±0.07; painless:0.84±0.15) and JNK-mRNA (painful:1.57±0.16; painless:1.05±0.21), but the significance of difference remained at 0.052 level for BACE1-mRNA (painful:1.60±0.13; painless:0.74±0.14). Figure 5
Figure 5.Pain perception prevents ERK1/2 phosphorylation and up-regulates PSEN2 mRNA levels in the rats with dentin erosion 60 min after HFS. A and B: The levels of total-ERK1/2 and phosphorylated ERK1/2 in the hippocampus of control rats (n = 6) and dentin erosive rats with molar teeth stimulated with warmish water (painless, n = 6) and cold water (painful, n = 6) were measured. Protein levels were detected by immunoblot analysis as described in the Methods section. Relative optical density (Y-axis) was calculated dividing the optical density of the analyzed band by that of own β-actin band, and quantified as percent change of the first control hippocampus sample on the membrane. *significant p values from Mann Whitney U test. C: Representative blots of total-MAPKs and p-MAPKs. Down-regulation of p-ERK1/2 in the blots of dentin erosive rats with molar teeth stimulated with warmish water (painless) is not seen in dentin erosive rats with molar teeth stimulated with cold water (painful). D: qRT-PCR analysis of neurodegeneration-related genes in LTP induced-hippocampus of control rats (n = 6) and dentin erosive rats with molar teeth stimulated with warmish water (painless, n = 6) and cold water (painful, n = 6). The cycle threshold (Ct) was determined for each target gene in duplicate. The β-Actin gene was used as the house- keeping gene (reference gene). At the end of the process, Ct (threshold cycle) values was recorded. The Ct values obtained were calculated and normalized using the 2−△△Ct method.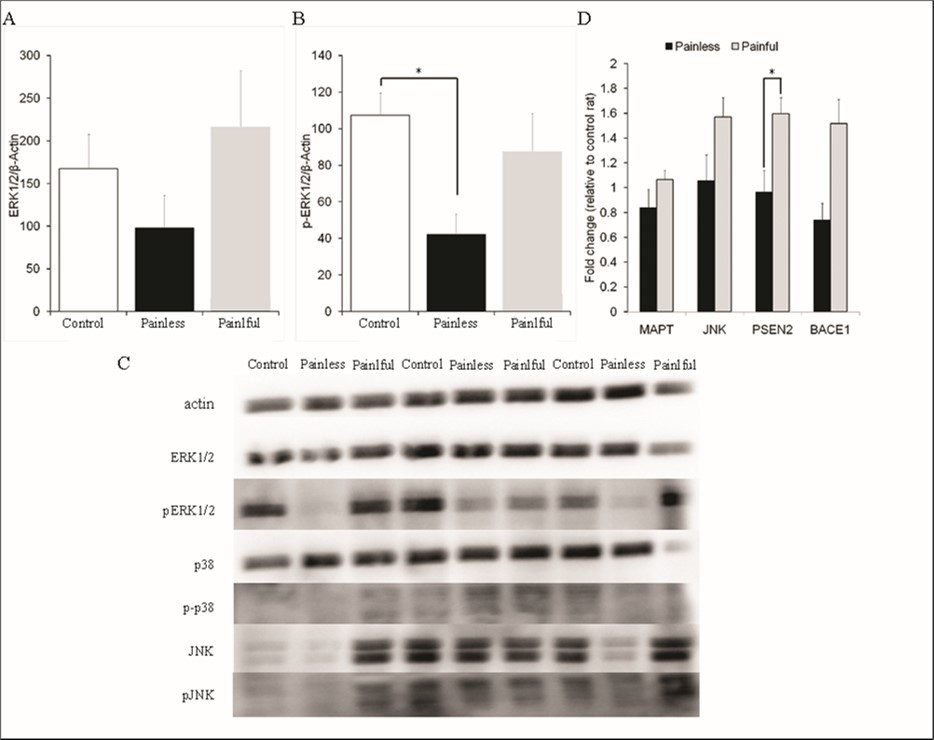
Discussion
LTP and LTD are synaptic plasticity types that characterize a potentiation or depression of the PS amplitude with or without fEPSP slope. The present study investigated whether pain sensation modulates neuronal plasticity. A bulk of finding from our study indicates that chronic and intermittent pain which is induced by dentin hypersensitivity facilitates the induction of plasticity. In control rats, neither LFS nor HFS resulted in a lasting change in synapse strength; however both stimulation methods induced somatic LTP. Interestingly, LFS was able to induce synaptic depression in the rats with dentin erosion. Moreover HFS-induced LTP increased in magnitude if the rats with dentin erosion were exposed to painful stimuli. The current findings suggest that pain sensation increases glutamatergic granule cell somatic plasticity after HFS but decreases it after LFS. In agreement with this finding, pain significantly increased LTP induced by theta burst stimulation in the DG and CA1 area of hippocampal slices of rats suffering from persistent nociception 50.
The present study used dentin hypersensivity model induced by dentin erosion mediated by acidic solution. Dentin hypersensitivity can be defined as a sharp, short pain arising from exposed dentin in response to thermal, tactile, osmotic, or chemical stimuli. According to the Hydrodynamic Theory, the pain of dentin hypersensitivity is caused by the quick movement of dentinal fluid which, in turn, excites the mechanoreceptors in the periphery of the pulp 5.In a functional magnetic resonance research, higher BOLD (blood-oxygen-level dependent) signals were identified in cortical areas involved in emotion and pain control when painful electrical stimulation was applied to maxillary canine teeth versus painless stimulation 7. As a result, it is possible to deduce that exposing teeth with compromised tissue integrity to cold stimulation causes a pain response. Such conclusions were supported by the analysis of hypersensitivity scores that shows cold stimulation caused increased motor response in the rats with dentin erosion.
The present study also investigated pain modulation of neuronal plasticity-related MAPK activity in experimental model of dental erosion. The present finding shows that peripheral tissue injury reduces ERK1/2 phosphorylation in the dentate gyrus, which is avoided by pain perception. It has been demonstrated that intra-plantar saline or bee venom injection, which simulate temporary or chronic pain, may cause a strong and long-lasting activation of ERKs in the hippocampus and primary somatosensory cortex21. Elk mRNA isoforms, a major target of ERKs within the nucleus, are individually up-regulated 2.3-fold in the dorsal root ganglia following peripheral nerve damage 25. Activation of the ERK isoforms of MAPK has been demonstrated to be necessary for the induction of NMDA receptor-dependent LTP in the dentate gyrus 9. Previous studies have provided evidence that chronic or persistent pain can cause malfunction in numerous brain structures involved in amnesia, insomnia, and depression 17, 13, 30. These findings support to the functional importance of ERKs-mediated signaling pathways in the processing of consequences of pain associated with cognitive dimensions.
We show that painful stimuli increase the expression of certain neurodegeneration-related genes, particularly PSEN2. PSEN1 and PSEN2 genes encode presenilins (presenilin 1 and presenilin 2) which constitute the catalytic subunits of the γ-secretase intramembrane protease protein complex. Mutations in presenilins cause autosomal dominant, early-onset familial AD (FAD) and promote cerebral Amyloid β accumulation 28. Transgenic mouse studies have revealed that FAD-linked presenilin 1 is associated with a greater degree of LTP induction in the hippocampus 35, 48, 38, 34, 12. To the best of our knowledge, no study has addressed a direct interaction between LTP, pain and presenilin 2. Our findings support the idea that a greater induction of LTP can be related with pain-induced up-regulation of PSEN2.
Previous studies have reported that many of patients with neurodegenerative disease such as Alzheimer’s disease 24, Parkinson’s disease 3 and Huntington’s disease 1 complain of painful symptoms though their origin is variable. The presented study draws attention to the increase in the transcription of some neurodegenerative proteins in the hippocampus tissue, which is involved in a number of neurodegenerative diseases, due to exposure to painful stimuli. Among these proteins, the pain-induced upregulation of PSEN2, which forms the catalytic subunit of the γ-secretase complex, during LTP induction seems to be important according to our study results. Other possible functions of presenilins are on important neuronal processes such as regulation of Ca homeostasis 8, modulation of apoptosis 11 and intracellular trafficking 33. It was also shown that presenilin expression is increased in the brains of some AD patients19, 42, 14. Therefore, our study highlights that exposure to painful stimulation has the potential to increase susceptibility to neurodegeneration accompanied by impaired regulation of plasticity.
The main limitation of the research is that it did not focus on the mechanism of which substance or substances mediate the modulation of plasticity by pain. A candidate mediator may be Orexin because the participation of Orexin receptors within the DG has been demonstrated in the anti nociceptive effects of lateral hypothalamic stimulation 6, a region that responds to both acute heat stimuli and a chronic inflammatory irritant 39. The DG region has the Orexin-containing axon terminals that come from neurons in the lateral hypothalamus 44. Orexin, when administered directly to the dentate gyrus, increased LTP, which was prevented by pretreatment with SB-334867, a selective Orexin 1 receptor antagonist 44. Exogenous orexin exacerbated hippocampal LTP depression, and increased Aβ and tau pathologies in APP/PS1/tau triple-transgenic AD model mice by affecting BACE1 29.
As a result, pain perception may influence synaptic and somatic neural plasticity, as well as ERK phosphorylation and PSEN2 expression in the dentate gyrus. Further investigations are needed to understand the molecular targets that mediate the pain modulation mechanism of plasticity. These studies will contribute to both a better understanding of the function of the hippocampus in the formation of memory traces of painful stimuli and to revealing the role of pathological pain processes in neurodegeneration.
Funding
This study was supported by Erciyes University Research Found (TCD-2020-10091)
Declarations
The authors declare that there is no conflict of interest.
References
- 1.Baez S, Herrera E. (2015) Impairments in negative emotion recognition and empathy for pain in Huntington's disease families.". , Neuropsychologia 68, 158-167.
- 3.A G Beiske, J H Loge. (2009) Pain in Parkinson's disease: Prevalence and characteristics.". , Pain 141(1), 173-177.
- 4.M R Bergamini, M Bernardi. (2014) Dentin hypersensitivity induces anxiety and increases corticosterone serum levels in rats.". , Life Sci 98(2), 96-102.
- 5.Brannstrom M, Astrom A. (1972) The hydrodynamics of the dentine; its possible relationship to dentinal pain.". , Int Dent J 22(2), 219-227.
- 6.M S Brojeni, Rashvand M. (2019) Role of orexin receptors within the dentate gyrus of the hippocampus in antinociception induced by chemical stimulation of the lateral hypothalamus in the tail-flick test as a model of acute pain in rats.". , Physiology & Behavior 209.
- 7.Brugger M, Lutz K. (2012) Tracing toothache intensity in the brain.". , J Dent Res 91(2), 156-160.
- 8.J D Buxbaum, E K Choi. (1998) Calsenilin: A calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment.". , Nature Medicine 4(10), 1177-1181.
- 9.A N Coogan, D M O'Leary. (1999) P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus in vitro.". , J Neurophysiol 81(1), 103-110.
- 10.S A Deadwyler, Dunwiddie T. (1987) A Critical-Level of Protein-Synthesis Is Required for Long-Term Potentiation.". , Synapse 1(1), 90-95.
- 11.Deng G, C J Pike. (1996) Alzheimer-associated presenilin-2 confers increased sensitivity to apoptosis in PC12 cells.". , FEBS Lett 397(1), 50-54.
- 12.Dewachter I, Ris L. (2008) Modulation of synaptic plasticity and Tau phosphorylation by wild-type and mutant presenilin1.". , Neurobiol Aging 29(5), 639-652.
- 13.B D Dick, Rashiq S. (2007) Disruption of attention and working memory traces in individuals with chronic pain." Anesth Analg 104(5): 1223-1229, tables of contents.
- 14.Diehlmann A, Ida N. (1999) Analysis of presenilin 1 and presenilin 2 expression and processing by newly developed monoclonal antibodies.". , J Neurosci Res 56(4), 405-419.
- 16.Engert F, Bonhoeffer T. (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity.". , Nature 399(6731), 66-70.
- 17.D A Fishbain, Cutler R. (1997) Chronic pain-associated depression: antecedent or consequence of chronic pain? A review.". , Clin J Pain 13(2), 116-137.
- 18.Galan A, Laird J M A. (2004) In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons.". , Pain 112(3), 315-323.
- 19.Giannakopoulos P, Bouras C. (1997) Presenilin-1-immunoreactive neurons are preserved in late-onset Alzheimer's disease." The American journal of pathology 150(2):. 429.
- 20.B J Gibson, O V Boiko. (2015) The everyday impact of dentine sensitivity: personal and functional aspects." Dentine Hypersensitivity: Developing a Person-Centred Approach to Oral Health:. 89-107.
- 21.Guo S-W, Liu M-G. (2007) Region-or state-related differences in expression and activation of extracellular signal-regulated kinases (ERKs) in naïve and pain-experiencing rats.". , BMC neuroscience 8(1), 1-14.
- 22.A J Heynen, B J Yoon. (2003) Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation.". , Nat Neurosci 6(8), 854-862.
- 23.Ji R-R, Kawasaki Y. (2007) Protein kinases as potential targets for the treatment of pathological pain.". , Analgesia: 359-389.
- 24.Kaufmann L, Moeller K. (2021) Pain and Associated Neuropsychiatric Symptoms in Patients Suffering from Dementia: Challenges at Different Levels and Proposal of a Conceptual Framework.". , J Alzheimers Dis 83(3), 1003-1009.
- 25.Kerr N, Pintzas A. (2010) The expression of ELK transcription factors in adult DRG: Novel isoforms, antisense transcripts and upregulation by nerve damage.". , Molecular and Cellular Neuroscience 44(2), 165-177.
- 26.Koga K, Shimoyama S. (2018) Chronic inflammatory pain induced GABAergic synaptic plasticity in the adult mouse anterior cingulate cortex.". , Mol Pain 14, 1744806918783478.
- 27.Korte M, Carroll P. (1995) Hippocampal Long-Term Potentiation Is Impaired in Mice Lacking Brain-Derived Neurotrophic Factor.". , Proc Natl Acad Sci U S A 92(19), 8856-8860.
- 28.H M Lanoiselee, Nicolas G. (2017) APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases.". , PLoS Med 14(3), 1002270.
- 29.Li Y, Yu K. (2023) Orexin-A aggravates cognitive deficits in 3xTg-AD mice by exacerbating synaptic plasticity impairment and affecting amyloid beta metabolism.". , Neurobiol Aging 124, 71-84.
- 30.Ling J, Campbell C. (2007) Short-term prospective memory deficits in chronic back pain patients.". , Psychosom Med 69(2), 144-148.
- 31.M G Liu, Chen J. (2009) Roles of the hippocampal formation in pain information processing.". , Neurosci Bull 25(5), 237-266.
- 32.Luo C, Kuner T. (2014) Synaptic plasticity in pathological pain.". , Trends Neurosci 37(6), 343-355.
- 33.Nishimura M, Yu G. (1999) Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of β-catenin, a component of the presenilin protein complex.". , Nature Medicine 5(2), 164-169.
- 34.Oddo S, Caccamo A. (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction.". , Neuron 39(3), 409-421.
- 35.Parent A, D J Linden. (1999) Synaptic transmission and hippocampal long-term potentiation in transgenic mice expressing FAD-linked presenilin 1.". , Neurobiol Dis 6(1), 56-62.
- 36.W J Ren, Liu Y. (2011) Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents.". , Neuropsy chopharmacology 36(5), 979-992.
- 38.Schneider I, Reverse D. (2001) Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation.". , Journal of Biological Chemistry 276(15), 11539-11544.
- 39.J N Siemian, M A Arenivar. (2021) An excitatory lateral hypothalamic circuit orchestrating pain behaviors in mice.". , Elife 10.
- 40.C H Splieth, Tachou A. (2013) Epidemiology of dentin hypersensitivity.". , Clin Oral Investig 17, 3-8.
- 41.P K Stanton, J M Sarvey. (1984) Blockade of Long-Term Potentiation in Rat Hippo campal-Ca1 Region by Inhibitors of Protein-Synthesis.". , Journal of Neuroscience 4(12), 3080-3088.
- 42.Takami K, Terai K. (1997) Expression of presenilin-1 and-2 mRNAs in rat and Alzheimer's disease brains.". , Brain research 748-1.
- 43.Tan B, Yasar A. (2022) Sex-related differences in somatic plasticity and possible role of ERK1/2: An in-vivo study of young-adult rats.". , Physiology & Behavior 255, 113939.
- 44.M J Wayner, D L Armstrong. (2004) Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo.". , Peptides 25(6), 991-996.
- 45.Wei F, Z C Xu. (2000) Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation.". , J Cell Biol 149(7), 1325-1334.
- 46.A J Whitmarsh. (2007) Regulation of gene transcription by mitogen-activated protein kinase signaling pathways.". , Biochim Biophys Acta 1773(8), 1285-1298.
- 47.W J Xin, Q J Gong. (2006) Role of phosphorylation of ERK in induction and maintenance of LTP of the C-fiber evoked field potentials in spinal dorsal horn.". , J Neurosci Res 84(5), 934-943.
- 48.S H Zaman, Parent A. (2000) Enhanced synaptic potentiation in transgenic mice expressing presenilin 1 familial Alzheimer's disease mutation is normalized with a benzodiazepine.". , Neurobiol Dis 7(1), 54-63.
