Effect of Solvent pH on Antioxidant and Phytochemical Activities of Mulhatti Aerial Parts (Glycyrrhiza glabra L.)
Abstract
Medicinal plants have been used since the era of Vedic history for their health care system where herbal medicine has a long history of use. It is also a very popular medicinal plant belonging to the Leguminosae family and is commonly known as Mulhatti. It contains phytochemicals such as flavonoids, triterpene saponins and other constituents such as coumarins, sugars, amino acids, tannins, starch, choline, phytosterols etc. The present study was conducted for the estimation of phytochemicals (total phenols and flavonoids) and the evaluation of the total antioxidant capacity and DPPH free-radical scavenging activity in aqueous extracts of different pH (2, 4, 7 and 9) from aerial parts of Glycyrrhiza glabra L. The content of phenolic compounds was maximal at pH 7 (14.13 mg GAE/g) and flavonoids at pH 9 (4.90 mg CE/g) and the total antioxidant capacity was maximal at pH 9 (13.43 mg AAE/g) and free radical scavenging DPPH activity was highest at pH 7 (IC50 value = 60.48 µg/ml). Thus, the aerial part is a good source of phytochemicals and also acts as a good antioxidant.
Author Contributions
Academic Editor: Kai Wang, No.1 Beigou Xiangshan, Haidian District, Beijing, China, 100093.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Parvesh Devi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
For their basic needs as a source of medicine, shelter, food, perfume, clothing, flavouring, fertilizer, and transportation, humans depend on nature. Medicinal plants have been used since the era of Vedic history. Especially in developing countries, large parts of the world's population depend on medicinal plants for their health care systems, where herbal medicines have a long history of use 1. Traditional medicine has existed in many countries in the world such as India, Pakistan and Bangladesh 2. Glycyrrhiza glabra L. it is also one of the most popular medicinal plants and belongs to the Leguminosae family and is commonly called Mulhatti. Glycyrrhiza glabra contains phytochemicals such as flavonoids (liquirtin, isoflavonoids and formononetin), triterpene saponins (glycyrrhizin, glycyrrhizin and liquirtic acid) and also contains other constituents such as coumarins, sugars, amino acids, tannins, starch, choline and phytosterols 3, 4. Mulhatti has antiviral, antitumor, antiulcer, antidiabetic, antioxidant, antithrombus, antimalarial, antimycotic, antibacterial, immunostimulant, antithrombotic, anticonvulsant, antiallergenic and expectorant activities 5, 6. Sandy clay soils are good for cultivation and, according to expert, the tropical climate of north-western India is suitable for watering, watering every 30-45 days in summer and little watering in winter. The lifespan of the plant is 2-3 years and the harvest is from November to December. The aim of this study was to determine the total phenolic content, flavonoids and total antioxidant capacity and free radical scavenging activity of DPPH from aqueous extracts with different pH (2, 4, 7 and 9) in the aerial part of Mulhatti.
Material and Methods
Plant Materials Collection
Mulhattiaerial parts (Glycyrrhiza glabra L.) were obtained from the experimental area of Medicinal, Aromatic & Potential Crops Section, Department of Genetics & Plant Breeding, Chaudhary Charan Singh Haryana Agricultural University, Hisar in 2017. Aerial parts of the herbarium were randomly collected from the field with a plant age of approximately 1.5 years and dried in a tunnel drying system during March. After drying, the samples were stored in a chemical laboratory. The sample was converted to powder form in a hammer mill.
Proximate Composition and Mineral Profile
Preliminary analysis of Mulhatti aboveground fractions is performed to determine water content, ash content, crude protein, total carbohydrate and calorific value according to standard methods specified in the Official Association of Analytical Chemists (AOAC) 7. The crude fibre content is estimated using a modified Maynard method 8.
Preparation of Plant Extracts
Five grams of Mulhatti's aerial part powder sample was packaged in a thimble made from Whatman No. 1 and extraction was carried out using a classic Soxhlet apparatus. The apparatus was provided with a 500 mL round bottom flask and distilled water as solvent was added to about half a siphon (240-270 mL) of different pH (2, 4, 7 and 9) and the pH was adjusted using HCl (conc.) and a NaOH pellet. Then the extraction is carried out at the boiling point of water still continue up to 5 to 6 cycles of Soxhlet extraction completed. The solvent vapour rises to the column and condenses in the condenser section of the apparatus. After condensation, they flow into a chamber thimble filled with a sample of Mulhatti air passages and the respective extracts are filtered. This process is repeated three times. The resulting filtrate was stored in bottles for further experiments.
Total Phenolic Content
The phenolic compounds present in aqueous extracts of the Mulhatti aerial parts at different pH (2, 4, 7 and 9) were estimated using the Folin-Ciocalteu method 9. To estimate the total phenols in aqueous extracts at various pHs; 0.2 mL of extract from each tube was diluted with the respective solvent to adjust the absorbance within the calibration limits. One mL of Folin-Ciocalteu reagent was added and 2 mL of Na2CO3 (20%, w/v) were mixed and the volume was made up to 10 mL with distilled water. The mixture was allowed to stand for 8 minutes and centrifuged at 6000 rpm for 10 min. Similarly, a blank is prepared; respective solvents were used instead of extracts. The absorbance of the supernatant solution was measured against a blank made on a UV-VIS Dual Ray Spectrophotometer (Shimadzu 1900) at 730 nm. The total amount of phenol present in aqueous extracts of different pH was calculated from the standard curve and expressed as mg GAE/g (milligram gallic acid equivalent per gram).
Flavonoids Content
The content of flavonoids present in aqueous extracts of the Mulhatti aerial parts at different pH (2, 4, 7 and 9) was estimated by the Aluminum chloride colorimetric method 10. To estimate the total flavonoids in aqueous extracts with different pH; 1 mL of each extract was taken into the tube and added 4 mL of distilled water, 0.3 mL of NaNO2 (5%) and added 0.3 mL of AlCl3 (10%) after 5 minutes and then 2 mL of NaOH (1 M) were added instantly and the final volume was made up to 10 mL with distilled water. Similarly, blank is prepared; respective solvents were used instead of the extracts. After the solution was thoroughly stirred, the absorbance was measured against a blank made on a UV-VIS dual beam spectrophotometer (Shimadzu 1900) at 510 nm. The quantity of flavonoids present in aqueous extracts of different pH was calculated from the standard curve and expressed as mg CE/g (milligram catechin equivalent per gram).
Total Antioxidant Capacity
Evaluating the total antioxidant capacity of aqueous extracts with different pH (2, 4, 7 and 9) of Mulhatti root using the modified phosphomolybdenum method 11. In glass vials, 0.3 mL of each extract was taken and 3 mL of phosphomolybdenum reagent was added and the solution was mixed thoroughly, the vial was closed with a cap. They were incubated for 90 minutes at 95°C. Next, the contents of the vial were allowed to cool and absorbance was measured at 695 nm on a UV-VIS model double beam spectrophotometer (Shimadzu 1900) against the prepared blanks. Similarly, blank is prepared; respective solvents were used instead of the extracts. The total antioxidant capacity in aqueous extracts of different pH was calculated from the standard curve and expressed as mg AAE/g (milligram ascorbic acid equivalent per gram).
Antioxidant Activity
Antioxidant activity was estimated by DPPH free radical scavenging activity method in aqueous extracts of Mulhatti aerial parts of different pH, namely 2, 4, 7 and 9 12. The aqueous extract was taken and dried completely and the dry mass was recorded. Aqueous extract was redissolved in an appropriate amount of 50% (v/v, methanol:water) to obtain a stock solution of 1000 g/mL depending on the dry weight of the extract (because it is not completely soluble in methanol). Concentrations from 5 g/mL to 500 g/mL were prepared from stock solutions (1000 g/mL) at appropriate dilutions with 50% (v/v) water:methanol. To evaluate the free radical scavenging activity of DPPH, 1 mL of extract of each concentration was put into a glass vial covered with a lid and 2 mL of 2,2'diphenyl1picrylhydrazyl (DPPH; 0.1 mM was prepared in 50% (v/v) water: methanol) and covered with a lid, shake well for 5 minutes.Control was carried out with 1 mL of each solvent used as a sample substitute. They were incubated for 30 min in the dark and then the absorbance of the sample and control was measured against a blank containing pure methanol at 517 nm on a UV-VIS Double Beam spectrophotometer (Shimadzu 1900) and each sample was brought to three replicas. Using Microsoft Excel software, graphs were plotted by plotting the free radical scavenging activity of DPPH (%) on the y-axis versus the extract concentration (µg/mL) on the x-axis, then the quadratic regression equation (y = ax² + bx + C). The obtained equation is converted into the form (ax² + bx + c = 0) by setting y = 50%. Using the equation (ax² + bx + c = 0), the IC50 value is calculated by applying the formula
x = 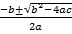
Where, x = IC50 (µg/mL) Percentage of activity eliminated by DPPH (% DPPH * SC) is calculated using
Where, Acontrol= controlabsorbance, Asample= sampleabsorbance
Statistical Analysis
All results are calculated in triplicate for their statistical study and expressed as mean ± SD. To assess a significant difference between the sample means in online statistical analysis (OPSTAT), one-way variances (ANOVA) were performed. IC50 values for the free radical activity of DPPH were calculated using a quadratic regression equation. The correlation between total phenolic compounds, total flavonoids and DPPH values for free radical scavenging and total antioxidant capacity were calculated using the Karl Pearson method in Microsoft Excel and all other measurements were also performed in Microsoft Excel 2019.
Results and Discussion
Compositional Profiling
The nutritional composition of Mulhatti aerial parts was determined by proximity method. This work was performed to evaluate the feasibility of using the above-ground parts of Mulhatti for medicinal purposes. Results of this study: Moisture, ash, crude fat, crude fiber, crude protein, total carbohydrate and calorific value were measured at 7.23 ± 0.15%, 6.82 ± 0.01%, 1.04 ± 0, 08% and 24.84 ± 0.54%, 15.84 ± 0.83%, 44.27 ± 1.19%, 249.96 ± 6.15 kcal in the aerial part of Mulhatti (Table 1). The results obtained for this analysis are similar to the reference data 13,14,15. The calorific value depends on the approximate composition of various plants.
Table 1. Proximate composition (%) of moisture, ash, fat, crude fibre, protein, total carbohydrates and calorific value (kcal) in aerial parts of Mulhatti| Parameters | Aerial parts |
|---|---|
| Moisture (%) | 7.23 ± 0.15 |
| Ash (%) | 6.82 ± 0.01 |
| Fat (%) | 1.04 ± 0.08 |
| Crude fibre (%) | 24.84 ± 0.54 |
| Protein (%) | 15.84 ± 0.83 |
| Total carbohydrates (%) | 44.27 ± 1.19 |
| Calorific value (kcal) | 249.96 ± 6.15 |
Total Phenolics
The amount of phenolic compounds present in the aqueous extract at different pH, namely 2, 4, 7 and 9 of arial parts of Mulhatti was determined using the equation (y = 0.0104x + 0.0079, R² = 0.9989) obtained from the acid calibration standard. Standard curve was used for the determination of amount of phenol and the total amount of phenol in mg GAE/g was determined. The study data for the amount of phenol did not show a regular trend but showed wide variation in the yield of aqueous extracts with different pH. The maximum amount of total phenolic content was present at pH 7 (14.13) followed by pH 2 (3.77), pH 9 (3.22) and pH 4 (2.60 mg GAE/g). Research data for phenolic compounds, flavonoid content and free radical scavenging activity of DPPH and total antioxidant capacity showed uneven trends at different pHs. Other researchers have also reported various effects of pH on phytochemical and antioxidant activity. In 2019, Krungkree and Arikul studied the effect of solvent pH on total phenolic content (TPC), DPPH radical scavenging, and their results showed that more phenolic compounds were present at pH 5-716. In general, TPC and antioxidant activity were higher as the pH increased. Therefore, the high amount of these phytochemicals at pH 5-7 indicates that the pH conditions have a significant effect on the stability of total phenols and the overall antioxidant capacity. Medina et al. (2007) also pointed out the reason for the high amount of total phenolic compounds at neutral pH, because hydrolysis of phenolic acids in alkaline and strongly acidic conditions reduces the amount of phenolic compounds 17. In sweet potato leaves, polyphenols exhibited high retention rates with an increase in acidity or alkalinity at pH 5-7, which had a significant effect on stability 18. Phenolic compounds extracted from Chamomile (Matricariapubescens) at different pHs namely: 3, 4, 5, 6 and 7 also showed variation, along with increasing pH from 3 to 5, the phenolic content also increased but after pH 5 the quantity decreased which indicated that the difference in the pH value of the extraction has a significant effect on the extraction of phenolic compounds 19.
Total Flavonoids
Similarly, the flavonoid content was also determined using the equation (y = 0.0018x + 0.0038, R² = 0.998) obtained from the calibration curve of the catechins used as standard and the amount of flavonoids was determined in mg CE/g. The results for flavonoids also show great variation. The maximum flavonoid content was found at pH 9 (4.90) followed by pH 7 (3.23), pH 2 (0.25) and pH 4 (0.24 mg EC/g). Flavonoids were determined from the top of the Schultz (Algerian MatricariaPubescens) and the results found to vary significantly at different pHs of the extraction solvent and increasing order were: pH 5 (6.36 ± 0.2 > pH 7 (4.88 ± 0.3) 12)> pH 6 (3.94 ± 0.17)> pH 4 (2.16 ± 0.17)> pH 3 (2.14 ± 0.19) 20.
Antioxidant Activity
To calculate the IC50 value of DPPH free radical scavenging activity, a quadratic equation was obtained by plotting the DPPH radical scavenging activity (%) on the y-axis and concentration (µg/mL) on the x-axis (Table 2) of Mulhatti aerial parts of aqueous extracts at different pH viz: 2, 4, 7 and 9.
Table 2. Quadratic regression equations for IC50 values (µg/mL) calculation of aqueous extracts of Mulhatti aerial parts at different pH (2, 4, 7 and 9)| pH | Aerial parts |
|---|---|
| 2 | y = -0.0005x2 + 0.4149x + 8.8039 R² = 0.9818 |
| 4 | y = -0.0006x2 + 0.4772x + 6.6752 R² = 0.9918 |
| 7 | y = -0.0021x2 + 0.8466x + 6.4812 R² = 0.9887 |
| 9 | y = -0.0008x2 + 0.5328x + 12.57 R² = 0.9561 |
The IC50 value of the Mulhatti aerial part in aqueous extracts at different pH (2, 4, 7 and 9) was lowest at pH 7 (60.48) followed by pH 9 (79.82), pH 4 (104.53), pH 2 (115.32 g/mL) (Table 3). Dogãn and Salman 2007 reported that the pH-dependent increase in the antioxidant activity of phenolic compounds was due to the greater stabilization in alkaline solutions which led to the polymerization reaction of the polyphenol antioxidants and which could form new moieties of oxidizable hydroxyl groups in the polymers 21. The product produces greater antioxidant activity than phenolic compounds.
Table 3. DPPH free radical scavenging activity (%) and IC50value (µg/mL) of aqueous extracts of Mulhatti aerial parts at different pH levels| pH | DPPH free radical scavenging activity at different concentration (µg/mL) | IC 50 (µg/mL) | ||||||
| 500 | 250 | 100 | 50 | 25 | 10 | 5 | ||
| 2 | 89.75 | 78.99 | 45.21 | 30.25 | 23.03 | 16.97 | A | 115.32 |
| 4 | 86.44 | 85.08 | 48.37 | 30.53 | 18.35 | 13.55 | 12.35 | 104.53 |
| 7 | A | 88.97 | 67.84 | 45.02 | 29.40 | 16.85 | 12.86 | 60.48 |
| 9 | 89.88 | 89.42 | 67.33 | 42.94 | 29.30 | 18.25 | 15.64 | 79.82 |
Total Antioxidant Capacity
Similarly, the total antioxidant capacity was determined using the equation (y = 0.0066x + 0.0036, R² = 0.999) obtained from the calibration curve of ascorbic acid used as a standard and the total antioxidant capacity was determined in mg AAE/g. The maximum total antioxidant capacity was found at pH 9 (13.43) followed by pH 2 (8.19), pH 7 (7.51) and pH 4 (5.87 mg AAE/g). Total antioxidant capacity by phosphomolybdenum method on dates (Phoenix Dactylifera L .) in aqueous extracts of different pH was evaluated and reported that the results showed no regular trend, the maximum antioxidant capacity was present at pH 4 (68.34 ± 0.71) followed by pH 6 (64. 12 ± 1.08), pH 5 (63.62 ± 0.69), pH 7 (62.81 ± 0.73), pH 3 (55.92 ± 0.60) and pH 2 (55.11 ± 0.60) 22, 23.
Free radical scavenging activities of phenol, flavonoid and DPPH in Thai curry paste extract at different pH (2 to 10) and the results showed a large variation as in this study and there was no correlation between the two. The number of phenolic compounds was higher in strong acids (pH 2) followed by slightly acidic (pH 6) and slightly alkaline (pH 10) and flavonoid compounds were present higher in strong acids (pH 2) and slightly acidic (pH 6). ) but the free radical scavenging activity of DPPH was found to be slightly higher in acid (pH 6) followed by pH 2, 3 and 10 respectively 24. As we know, this large variation in yield may be due to the types of samples used and the different types of compounds extracted in each case and the different phytochemical compositions of the plants depending on their different properties such as waxy or non-waxy species, plant parts used i.e. leaves, stems, bark, roots, tops or whole plants for research and also depending on the selection of compounds such as carotenoids, non-polar compounds, polar compounds and simple phenolic compounds also act as determinants of factors causing differences in phytochemical composition. Pearson correlation coefficient analysis was carried out to study the significant and insignificant correlation between the total phenol content, flavonoid and IC50 scavenging activity of DPPH and between the total phenol content, flavonoid and total antioxidant capacity. Pearson correlation coefficient is negative significant if 0.61 ≤ r ≤ -0.97 and significant positive if 0.61 ≤ r ≤ 0.97 25. Graphically, data for total phenolics, flavonoids and for total antioxidant capacity has shown below in figure 1 and Figure 2.
Figure 1.Image A of Mulhatti (Glycyrrhiza glabra) showed complete plant and B showed aerial parts
Figure 2.Effect on total phenolics, flavonoids and total antioxidant capacity of aerial parts of Mulhatti in aqueous extracts of different pH levels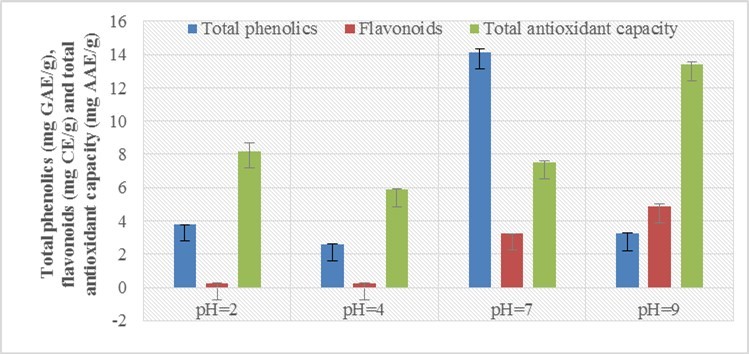
This study showed that the total phenol and flavonoid content had a significant and negative correlation with IC50 scavenging activity of DPPH (r = -0.782 and r = -0.794, P<0.05) and that phenolic compounds and flavonoids could be predicted as the main contributors in antioxidant activity by the DPPH method. This study also revealed that flavonoids had a significant and positive correlation with their total antioxidant capacity (r = 0.799, P < 0.05) and indicated that flavonoid compounds were the main contributors to total antioxidant capacity by the phosphomolybdenum method 26.
Conclusions
From this study, we can conclude that the phytochemical and free radical scavenging activities of DPPH and total antioxidant capacity were significantly affected by the aqueous extracts of different pH: 2, 4, 7 and 9 and the results of the research data clearly showed differences in the amount of phenolic compounds, flavonoids and antioxidants. Total antioxidants capacity and DPPH of free radical scavenging activity was shown by aqueous extracts with different pH levels. The highest phenol content was present at pH 7 and the highest flavonoid was present at pH 9. Therefore, pH 7 was the best for studying phenolic compounds and pH 9 was an excellent factor for determining flavonoid compounds in the aerial part of Mulhatti at different pH of aqueous extracts. The pH 9 is better to confirm the total antioxidant capacity of Mulhatti's aerial parts. The phenolic compounds and flavonoids present are responsible for the antioxidant activity and show a correlation with the free radical scavenging activity of DPPH and flavonoid compounds also show a correlation with the total antioxidant capacity. As the present study provides strong evidence of high total phenolic content and significantly higher free radical scavenging activity at pH 7 of the aqueous extracts.
References
- 1.WHO. (1998) Regulatory situation of herbal medicines. A worldwide review. Pp 1-5. , Geneva, Switzerland
- 2.Pk M, Wahile A. (2006) Integrated approach towards drug development from Ayurveda and other Indian systems of medicine.J. 103, 25-35.
- 3.T P Arystanova, M P Irismetov, A O Sopbekova. (2001) Chromatographic determination of glycyrrhizinic acid in Glycyrrhiza glabra preparation.Chemistry of Natural Compounds.1(37):. 89-90.
- 4.Fukai T, B S Cai, Maruno K, Miyakawa Y, Konishi M et al. (1998) An isoprenylated flavanone from Glycyrrhiza glabra and rec-assay of licorice phenols.Phytochemistry.49(7):. 2005-2013.
- 5.Z G Balasaheb.Pharmacological studies of taverniera cuneifolia Roth Arn a substitute for commercial liquorice.
- 6.Sahu Y, J S Vaghela. (2011) Protective effects of some natural and synthetic antidepressants against chronic fatigue induced alterations.J Global Pharma. Technol.3: 21-30.
- 7.Horwitz W, Chichilo P, Reynolds H. (1970) Official methods of analysis of the Association of Official Analytical Chemists. Official methods of analysis of the Association of Official Analytical Chemists.
- 9.V L Singleton, J A Rossi. (1965) Colorimetry of total phenolics with phosphor molybdic-phosphotungstic acid reagents. , American journal of Enology and Viticulture 16(3), 144-158.
- 10.Ribarova F, Atanassova M. (2005) Total phenolics and flavonoids in Bulgarian fruits and vegetables. , Journal of the university of chemical technology and metallurgy 40(3), 255-260.
- 11.Prieto P, Pineda M, Aguilar M. (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry. 269(2), 337-341.
- 12.Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T et al. (2000) Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus.Chemical and Pharmaceutical Bulletin.48(9):. 1286-1292.
- 13.Radha R, Rajaathi G. (2015) Phytochemical analysis of Glycyrrhiza glabra. , Indian J. Sci 22, 59-68.
- 14.W A Helmy, Z A El-Kheir, M S Abdel-Hady, El-Hameid A R A. (2013) Biological activities of aqueous extracts and their sulfated derivatives from licorice (Glycyrrhiza glabra L.). , Journal of Applied Sciences Research 9(6), 3638-3645.
- 15.S E Badr, D M Sakr, S A Mahfouz, M S Abdelfattah. (2013) Licorice (Glycyrrhiza glabra L.): Chemical composition and biological impacts. , Research Journal of Pharmaceutical, Biological and Chemical Sciences 4(3), 606-621.
- 16.Krungkri W, Areekul V. (2020) Effect of Heating Condition and pH on Stability of Total Phenolic Content and Antioxidant Activities of Samui (Micromelum Minutum) Extract.
- 17.Medina I, J M Gallardo, M J González, Lois S, Hedges N. (2007) Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. , Journal of Agricultural and Food Chemistry 55(10), 3889-3895.
- 18.H N Sun, T H Mu, L S Xi. (2017) Effect of pH, heat, and light treatments on the antioxidant activity of sweet potato leaf polyphenols. , International Journal of Food Properties 20(2), 318-332.
- 19.Altunkaya A, Gökmen V, L H Skibsted. (2016) pH dependent antioxidant activity of lettuce (L. sativa) and synergism with added phenolic antioxidants.Food chemistry.190:. 25-32.
- 20.L S Eddine, Djamila B, O M Redha. (2016) Solvent pH extraction effect on phytochemical composition and antioxidant properties of Algerian Matricaria Pubescens. , Journal of Pharmacy Research 10(2), 106-112.
- 21.Doğan S, Salman Ü. (2007) . Partial characterization of lettuce (Lactuca sativa L.) polyphenol oxidase.European Food Research and Technology.226(1): 93-103.
- 22.Zohra R, O M Redha, L S Eddine. (2016) Evaluation of phenolic content and antioxidant capacity of leaf extract from Phoenix Dactylifera L. obtained by different pH of aqueous extraction.Journal of Pharmacy Research.10(1):. 1-7.
- 23.L S Eddine, Djamila B, O M Redha. (2016) Solvent pH extraction effect on phytochemical composition and antioxidant properties of Algerian Matricaria Pubescens. , Journal of Pharmacy Research 10(2), 106-112.
- 24.Settharaksa S, Jongjareonrak A, Hmadhlu P, Chansuwan W, Siripongvutikorn S. (2012) Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature.International Food Research Journal.19(4).
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
International Journal of Food Science & Technology (2024) OpenAlex
International Journal of Food Science and Technology (2024) Crossref
Akram A. Qasem, I. M. Mohamed Ahmed, Belal M Mohammed, C. Brennan, Mehmet Musa Özcan et al. - International Journal of Food Science & Technology (2024) Semantic Scholar

